Children’s National Research Institute | Academic Annual Report 2017-2018
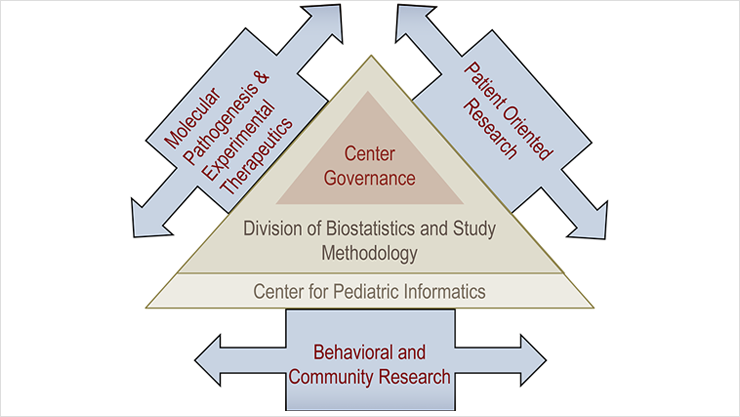
Innovation Through Collaboration
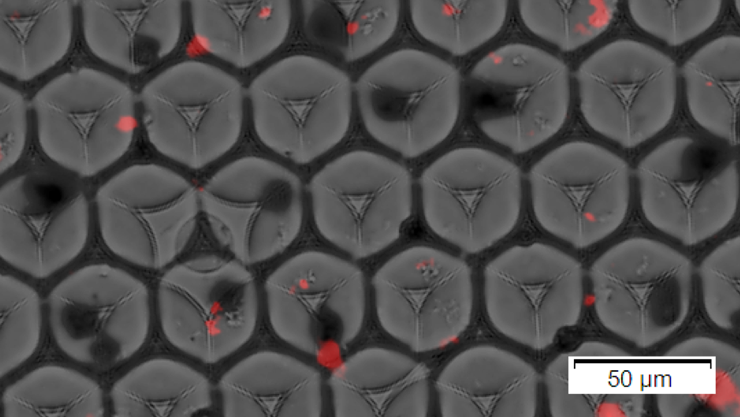
Beads (black) and synaptosomes (red) encapsulated in oil droplets.
Center for Neuroscience Research
Vision: The Center for Neuroscience Research seeks to understand the cellular, molecular, synaptic, and network mechanisms of brain development and dysfunction to prevent or treat developmental, neurological and behavioral disorders of childhood.
The Center for Neuroscience Research (CNR) comprises an expanding group of highly productive lab-based developmental neuroscientists and clinical investigators leveraging established strong research programs and successful collaborations to investigate neurodevelopmental disorders. While each investigator has distinct expertise and research interests, research in CNR as a whole is focused on development of the nervous system in health and in childhood neurological disorders. Programs range from those investigating early stages of nervous system formation, to work on postnatal processes such as synapse formation and the wrapping of neuronal processes by the myelin. The extraordinary and exciting setting of the center supports and promotes a large number of research projects that span basic, translational and clinical research in neurodevelopmental disorders.
Leadership
- Joshua Corbin, Ph.D., Interim Director
- William D. Gaillard, M.D., Associate Director
Faculty List
- Maria T. Acosta, M.D. Neurology
- Madison M. Berl, Ph.D. Neuropsychology
- Jessica Carpenter, M.D. Epilepsy, Neurophysiology, Critical Care Neurology
- Taeun Chang, M.D. Epilepsy, Neurophysiology, Critical Care Neurology
- Li-Jin Chew, Ph.D. Developmental Neurobiology
- Cedric Clouchoux, Ph.D. Diagnostic Imaging and Radiology
- Joan Conry, M.D. Epilepsy, Neurophysiology, Critical Care Neurology
- Julia Dorfman M.D., Ph.D. Psychology
- Adré Du Plessis, M.B.Ch.B. Fetal and Transitional Medicine
- Gerard Gioia, Ph.D. Neuropsychology
- Penny Glass, Ph.D. Psychology
- Rathinaswamy Govindan, Ph.D. Gastroenterology, Hepatology, and Nutrition
- Andrea Gropman, M.D. Neurology, Developmental Pediatrics
- Kristina Hardy, PsyD Neuropsychology
- Kazue Hashimoto-Torii, Ph.D. Developmental Neurobiology
- Anne Pradella Inge, Ph.D. Neuropsychology
- Nobuyuki Ishibashi, M.D. Cardiovascular Surgery
- Beata Jablonska-Gierdalska, Ph.D. Developmental Neurobiology
- Jyoti Jaiswal, Ph.D. Developmental Neurobiology (Joint membership with Center for Genetic Medicine)
- Richard A. Jonas, M.D. Cardiac Surgery
- Parmajit T. Joshi, M.D. Psychiatry
- Lauren Kenworthy, Ph.D. Neuropsychology
- Tarannum Lateef, M.D. Neurology
- Catherine Limperopoulos, Ph.D. Diagnostic Imaging and Radiology, Fetal and Transitional Medicine
- Judy S. Liu, M.D., Ph.D. Developmental Neurobiology, Epilepsy
- Dilip Nath, M.D. Cardiovascular Surgery
- An Nguyen-Massaro, M.D. Neonatology
- Nickie Niforatos, M.D. Neonatology, Fetal and Translational Medicine
- Chima Oluigbo, M.D. Neurosurgery
- Roger J. Packer, M.D. Neurology
- Anna Penn, M.D., Ph.D. Neonatology, Fetal and Transitional Medicine, Developmental Neurobiology
- Cara Pugliese, Ph.D. Neuropsychology
- Jacqueline Sanz, Ph.D. Neuropsychology
- Joseph Scafidi, M.D. Critical Care Neurology, Developmental Neurobiology, Epilepsy, Neurophysiology
- Billie Lou Short, M.D. Neonatology
- Michael Shoykhet, M.D., Ph.D., Critical Care Neurology
- Nathan Smith, Ph.D., Developmental Neurobiology
- John Strang, Psy.D. Neuropsychology
- Laura Tochen, M.D. Neurology
- Masaaki Torii, Ph.D. Developmental Neurobiology
- Jason Triplett, Ph.D. Developmental Neurobiology
- Tammy N. Tsuchida, M.D., Ph.D. Epilepsy, Neurophysiology, Critical Care Neurology
- Christopher Vaughan, Psy.D. Neuropsychology
- L. Gilbert Vezina, M.D. Radiology
- Karin Walsh, PsyD Neuropsychology
- Steven Weinstein, M.D. Epilepsy, Neurophysiology, and Critical Care Neurology
- Elisabeth Wells, M.D. Neurology
- Irene Zohn, Ph.D. Developmental Neurobiology
- Zungho Zun, Ph.D. Diagnostic Imaging and Radiology, Fetal and Transitional Medicine
Center Research Programs
Developmental Neurobiology
Neural Stem Cells
- Joshua Corbin, Ph.D.
- Vittorio Gallo, Ph.D.
- Nobuyuki Ishibashi, M.D.
- Beata Jablonska, Ph.D.
- Richard Jonas, M.D.
- Joseph Scafidi, M.D.
Dr. Gallo studies cellular signals that regulate the development of neural stem cells and progenitors in the perinatal and adult brain. His laboratory has extended these studies to animal models of brain injury and disease, including demyelinating disorders in a number of conditions including perinatal hypoxia and Down syndrome, the latter of which is supported by a new NIH RO1 from NINDS in collaboration with the laboratory of Dr. Tarik Haydar at Boston University to study mechanisms underlying dysregulation of white matter development in Down syndrome.
Drs. Ishibashi, Jonas and Gallo study neural stem cell development in the porcine brain, which closely resembles the human brain. Dr. Ishibashi found that the porcine subventricular zone (SVZ) shares the same cellular structure as its human counterpart at a comparable developmental stage. These similarities strongly support the notion that studies carried out in the porcine SVZ will provide novel insights into cellular/molecular and developmental mechanisms that are also relevant to the human SVZ under both normal physiological and pathological conditions. The team’s analysis revealed that chronic hypoxic exposure severely impairs neurogenesis within the porcine SVZ, resulting in a depletion of a critical source of interneurons destined to populate and potentially fine-tune the postnatal cortex. In addition, the team identifies that this pathology limits cortical expansion and gyrencephaly and mirrors the brain signatures seen in patients with congenital heart disease.
Dr. Jablonska studies cell cycle mechanisms involved in neural progenitor response to injury and their potential to regenerate glia, in particular intrinsic cell cycle regulatory mechanisms that modulate progenitor cell proliferation after injury. Growth factors and their corresponding receptors play important roles at critical time points in the developing postnatal brain. Brain cancers are examples of abnormal regulation of these growth factor signaling pathways. Some approaches to cancer therapy are to target these aberrant signaling pathways in neural stem/progenitor cells. Dr. Jablonska identified the histone deacetylase Sirt1 as a critical regulator of progenitor cell proliferation induced by perinatal brain injury.
Dr. Scafidi, with the support of the Childhood Brain Tumor Foundation and the National Brain Tumor Society, studies the effects of molecularly targeted therapies on stem/progenitor cells in different brain regions during normal development, including the hippocampus. Using genetic fate-mapping techniques, cellular imaging, behavioral studies and physiology, he is continuing to assess whether these therapeutic agents affect brain function and whether their effects are age dependent.
Myelin and White Matter Development
- Li-Jin Chew, Ph.D.
- Vittorio Gallo, Ph.D.
Drs. Gallo and Chew continue to study new cellular and molecular approaches that promote oligodendrocyte maturation, myelination, and white matter development. Dr. Chew is studying signal transduction pathways that regulate oligodendrocyte development in cultured cells and in transgenic mice. The focus of these studies is on mechanisms that promote oligodendrocyte progenitor differentiation and developmental myelination under pathological conditions. Dr. Gallo continues to study oligodendrocyte progenitor cell migration during normal development and after white matter injury. A focus of Drs. Gallo and Chew’s research is the function of Sox transcription factors—in particular Sox17— in oligodendrocyte development and pathology. The team identified downstream signaling pathways of Sox transcription factors that are involved in regulating specific phases of oligodendrocyte development and myelination.
Additionally, Dr. Chew studies how inflammation impacts oligodendrocyte progenitor cell function in cellular maturation, myelin gene expression, and repair after demyelination injury. By understanding the effects of chronic inflammation on the progenitor cells of developing white matter and in white matter lesions, it is hoped that therapeutic targets may be identified for strategies of pharmacological intervention.
Cerebral Cortex Development and Epilepsy
- Masaaki Torii, Ph.D.
- Kazue Hashimoto-Torii, Ph.D.
- Nathan Smith, Ph.D.
Both genes and environment influence development of the cerebral cortex, the brain region sub serving higher intellectual functions. Moreover, genetic abnormalities including disorders initiated by single gene mutations cause a large proportion of intellectual disabilities. Cognitive function in many of these disease states is altered in large part through disruption of proper prenatal development of the cerebral cortex. More specifically, loss of the proper migration, morphology and connectivity of cortical neurons results in intellectual disability and epilepsy.
Dr. Torii’s lab works to decipher the complex mechanisms in which these factors impact normal development of the brain, and to translate the findings into the development of novel therapeutic approaches for neurodevelopmental disorders such as schizophrenia and autism.
Toward this goal, the lab uses combinations of cutting-edge tools and techniques, including in vivo gene manipulation, induced pluripotent stem (iPS) cells, transgenic animals and animal disease models, proteomic and transcriptomic analyses, and cell encapsulation and transplantation. Preliminary studies in Dr. Torii’s lab have identified genes and their novel roles in regulating the number of neuronal connections across of the corpus callosum, the largest white matter bundle connecting left and right cortical hemispheres. This exciting work is supported by a newly funded NIMH RO1 to study the role of autism linked genes in developmental refinement of the corpus callosum. These findings can possibly identify the key mechanisms responsible for anatomical and functional abnormalities in this structure in various neurodevelopmental disorders. The research in the Torii lab is also supported by the Scott-Gentle Foundation.
Harmful conditions, such as hypoxia, exposure to excessive levels of heavy metals, and maternal smoking and alcohol intake are thought to reprogram normal fetal brain development and consequently increase the incidence of many childhood disorders, including lower birth weight, sudden infant death syndrome (SIDS), pediatric epilepsy, schizophrenia and ADHD. However, molecular mechanisms underlying such reprogramming remain obscure. Dr. Hashimoto-Torii’s laboratory seeks to understand how an adverse prenatal environment interacts with genetic predisposition, thereby increasing disease susceptibility after birth. With a focus on maternal alcohol drinking, the team tackles this question through a combination of wet and dry analyses using mouse and human research models (supported by NIH/ National Institute on Alcohol Abuse and Alcoholism).
One new major direction is to establish biomarkers for early detection of cognitive deficits in individuals with fetal alcohol syndrome. This new work is supported by a newly funded U award as part of a Collaborative Research at the Clinical Center to identify novel biomarkers for intellectual disability in children prenatally exposed to alcohol.
In addition, the lab is also testing potential drugs and devices to improve behavior problems of offspring after in utero exposure to harmful agents (supported by Scott-Gentle foundation).
Projects in Dr. Smith’s laboratory focus on “Neuro-Glia” interactions. The lab explores the understudied and novel mechanisms by which neuromodulators mediate the interactions between neurons, astrocytes, and microglia in both normal and disease states. By studying how neuromodulators mediate the unique interactions between these three cell types, the team will elucidate their coordinated functions in the normal, healthy brain and study how disruptions of neuronal glial crosstalk contribute to disease processes such as ADHD, depression and epilepsy. To accomplish these goals, the lab employs a combination of transgenic mouse lines, electrophysiology, pharmacology, behavioral assays and 2-Photon Ca2+ imaging in acute slices and awake behaving animals.
Structural Birth Defects
The structural birth defects program focuses on elucidating how genes interact with environmental factors to cause a variety of birth defects including spina bifida, anencephaly, dysphagia, and cardiac, placental and vertebral defects.
- Irene Zohn, Ph.D.
- Kelsey Sugrue, Ph.D.
- Bethany Stokes, M.S.
Structural birth defects occur in approximately 6 percent of births worldwide and are the leading cause of death for infants in the first year of life. While some birth defects are inherited in a Mendelian fashion, the majority of birth defects are due to complex interaction of poorly understood genetic factors with genetic or environmental modifiers. Because of the complex nature of these defects, the genetic causes are rarely known providing a significant challenge for genetic counseling and treatment. Research in the laboratory aims to fill this gap in knowledge by providing a mechanistic understanding of how genetic susceptibility loci interact with modifying genetic and environmental factors to cause structural birth defects. Using mouse models of structural birth defects, the team studies the developmental mechanisms that results in birth defects and how these processes can be modulated by micronutrients in the maternal diet such as folic acid, iron, and Vitamin A. While a genetically normal embryo will show normal development in spite of mild deficiency or excess of these nutrients, the team’s research indicates that some genetic backgrounds are unable to buffer small changes in micronutrients resulting in greater susceptibility to birth defects. Other genes studied play key roles in uptake or metabolism of these micronutrients and birth defects result with mild vitamin deficiencies. The team models these interactions in the mouse and collaborates with geneticists from regions of the world where vitamin deficiencies are common to identify mutations in these genes associated with birth defects. Dr. Zohn has obtained funding from NIH, March of Dimes and Spina Bifida Association for this research.
Development and Dysfunction of the Social Brain
- Joshua Corbin, Ph.D.
The amygdala regulates specific aspects of emotional memory, attention to socially salient stimuli and appropriate responses to these stimuli. The laboratory of Dr. Corbin studies the link between embryonic neurodevelopmental gene regulation and the formation of amygdala circuitry and related emotional and social behaviors. The lab also models the underlying defects in these processes that occur in developmental disabilities, such as autism spectrum disorders. Using animal models of amygdala development and malformation, the team has recently identified specific genetic mechanisms that underlie the formation of complex amygdala neural circuits. Dr. Corbin’s lab has also been interested in the function of amygdala interconnected brain regions such as the hypothalamus, a major hormonal integrative center for mediating social and non-social behaviors. Recent work uncovered a developmental genetic basis for hypothalamic development and control of stress-related behavior. Additionally, working in collaboration with BioPharma, Dr. Corbin and his team have revealed potential avenues of pharmacological intervention for social deficits associated with autism spectrum disorders, such as fragile X syndrome. The lab is also leveraging knowledge of the genetics of amygdala development to establish new biomarker tools to categorize individuals with autism based on biological criteria. The Corbin lab is funded by the NIH with previous funding from Autism Speaks and the Fragile X Foundation.
Sensory System Development
- Jason Triplett, Ph.D.
Deficits in sensory function are prevalent in neurodevelopmental disorders (NDDs), such as autism and fragile X syndrome, presenting an attractive target for therapeutic intervention. However, our understanding of how sensory circuit development goes awry is limited, as is our knowledge of the normal developmental trajectory. Research in Dr. Triplett’s lab is focused on understanding the mechanisms underlying sensory system development, organization and function. Using genetic, anatomical, molecular, and physiological approaches, the lab has uncovered fundamental principles governing the formation of sensory maps of space and the establishment of precise connections in the brain. In addition to investigating these basic mechanisms, the lab is currently elucidating alterations in circuit development and function associated with NDDs. Together, this knowledge is critical for the development of novel therapeutic targets for intervention.
Developmental Disabilities
District of Columbia Intellectual and Developmental Disabilities Research Center (DC-IDDRC)
- Vittorio Gallo, Ph.D.
- William D. Gaillard, M.D.
- Madison M. Berl, Ph.D.
- Joshua Corbin, Ph.D.
- Lisa Guay-Woodford (Center for Translational Science)
- Jyoti Jaiswal, Ph.D. (Center for Genetic Medicine Research)
- Susan Knoblach, Ph.D. (Center for Genetic Medicine Research)
- Catherine Limperopoulos, Ph.D. (Fetal Medicine Institute)
Initially established and funded in 2001, the District of Columbia Intellectual and Developmental Disabilities Research Center (DC-IDDRC) at Children’s National Hospital was re-funded by the National Institute of Child Health and Human Development (NICHD) in 2016. Directed by Dr. Gallo with associate director, Dr. Gaillard, the center supports five scientific core resources used by more than 70 National Institutes of Health (NIH)-funded investigators studying brain development and function, as well as various aspects of neurodevelopmental disorders, at Children’s National, George Washington University (GW), Howard University and Georgetown University. The center also supports a research project on premature cerebellar injury led by Dr. Limperopoulos.
The activities of IDDRC investigators span multiple areas of research corresponding to different IDD-associated conditions including brain injury, autism, urea cycle disorders and white matter disorders. In each of these areas, genetic, translational neuroscience and behavioral science programs are integrated to provide a multidisciplinary approach to each research theme. The areas of research are supported by Children’s National infrastructure and by the following scientific cores: Clinical and Translational, Genomics and Proteomics, Cell and Tissue Imaging, Neuroimaging, and Neurobehavioral Evaluation. Each of these cores has grown based on steady institutional investment in infrastructure, personnel, state-of-the-art equipment, and software. The Cell and Tissue Imaging, Neuroimaging, and Neurobehavioral Evaluation cores are all part of the Center for Neuroscience Research and are directed by Drs. Jaiswal, Gaillard and Berl, and Corbin, respectively.
Brain Injury and Brain Protection
- Gerard Gioia, Ph.D.
- Adré Du Plessis, MBChB
- Vittorio Gallo, Ph.D.
- Andrea Gropman, M.D.
- Nobuyuki Ishibashi, M.D.
- Richard Jonas, M.D.
- Catherine Limperopoulos, Ph.D.
- An Nguyen-Massaro, M.D.
- Joseph Scafidi, M.D.
- Anna A. Penn, M.D., Ph.D.
- Nickie Niforatos, M.D.
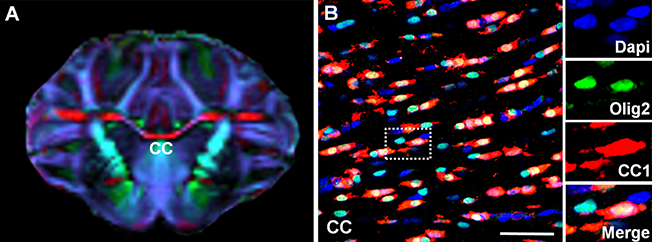
(A) Porcine white matter structures on diffusion tensor imaging. (B) Olig2+ porcine oligodendrocytes and CC1+ mature oligodendrocytes in corpus callosum (CC). Fractional anisotropy (FA) enables capturing cellular events 4 weeks after pediatric cardiac surgery. The risk of surgery-induced FA reduction due is primarily minimized by maintaining mature oligodendrocytes. (Ishibashi lab)
Traumatic brain injury (TBI) is the leading cause of acquired brain damage in children, producing persistent functional disability. The response to, and recovery from, TBI differs in adults and children. Brain damage from TBI is determined not only by direct mechanical injury to neural structures, but also by delayed axonal degeneration and neuronal cell death (apoptosis). Dr. Gioia’s research team employs multi-center TBI collaborations. His concussion symptom tool has facilitated collaborative national and international projects, including two Canadian national projects and a National Emergency Department concussion screening project partnering with Cerner and Epic to use electronic health records as data collection methodology with an associated national registry. The team also evaluates the effect of concussion on everyday living and quality of life, the effect of concussion on white matter microstructure, and the use of sensors to determine strain during sports head injury.
Dr. Gropman leads efforts to combine translational research with personalized and integrative medicine to alleviate the debilitating symptoms of pediatric mitochondrial diseases and urea cycle disorders.
Dr. Massaro continues her investigations of biomarkers of hypoxic ischemic brain injury (HIE). The Children’s National NICU played a key role in the phase II erythropoietin trial for brain protection, with Drs. Chang and Massaro as lead investigators.
Dr. Du Plessis, chief of Fetal and Transitional Medicine, along with Dr. Limperopoulos, director of Radiology and Neuroimaging Research, continue to expand their imaging of congenital malformations with a particular focus on cerebellar development. They are mapping the trajectory of fetal brain development with advanced imaging techniques. Important technical advances have been achieved to improve motion correction, which is necessary to optimize fetal brain imaging.
Dr. Penn focuses on the role of placental function in preterm brain injury and protection. Using a novel mouse model, she is investigating the impact of placental allopregnanolone loss and replacement on cortical and cerebellar development and injury. Translational studies measuring the same hormone in human newborns and placentas are underway.
Drs. Jonas and Ishibashi, in collaboration with Dr. Gallo, continue their investigations of neuroprotection in children with congenital heart disease, with an emphasis on white matter injury prevention. They have demonstrated prolonged microglia activation in white matter after cardiac surgery, identifying that controlling this phenomenon is a potential therapeutic intervention to limit neurological deficits following cardiac surgery. They also demonstrated selective vulnerability of pre-oligodendrocytes among different developmental stages, whereas oligodendrocyte progenitor cells were resistant to insults associated with cardiac surgery. The studies consistently support the concept that optimal treatment to achieve successful white matter development in children with congenital heart disease requires therapy aimed at promoting endogenous recovery through the action of oligodendrocyte progenitor cells. This work is supported by a newly funded NIH RO1 from National Heart, Lung and Blood Institute to study cell therapy for neuroprotection in congenital heart disease.
Board of Visitors Perinatal Neuroprotection Program
- Vittorio Gallo, Ph.D.
- Anna A. Penn, M.D., Ph.D.
- Billie Lou Short, M.D.
- Taeun Chang, M.D.
- An Nguyen-Massaro, M.D.
- Nickie Niforatos, M.D.
- Adré Du Plessis, MBChB
- Catherine Limperopoulos, Ph.D.
In the U.S., cerebral palsy (CP) is the most common motor disability of childhood. There are approximately 10,000 new patient diagnoses each year—the majority related to developmental brain injury in former premature infants. Children’s National has established a Cerebral Palsy Prevention Program, generously supported by the Board of Visitors as well as by external funding from the Cerebral Palsy Alliance. This year the program has been renamed to emphasize the institution’s strong focus on prevention of brain injury, specifically in the perinatal period, with the long-term goal of preventing the development of CP. Led by Drs. Gallo and Penn, the program’s transdisciplinary team has established new protocols for care of the most fragile preterm infants as part of the Preterm NeuroNICU cohort, creating a framework for future clinical trials. Children’s hosts national CP research leaders for a bi-annual lecture series and are engaged with communities and foundations that support CP research. In the laboratory, the team continues to expand investigations of endogenous neuroprotective agents that patients are missing due to preterm birth. Through clinical and research CP fellowships, Children’s is training the next generation of CP researchers to work together using multiple perspectives—clinical, bench-based and translational—to improve the developmental outcomes of preterm infants.
Perinatal Hypoxia and Hyperoxia
- Li-Jin Chew, Ph.D.
- Vittorio Gallo, Ph.D.
- Beata Jablonska, Ph.D.
- Joseph Scafidi, M.D.
- Nobuyuki Ishibashi, M.D.
- Richard A. Jonas, M.D.
- Anna Penn, M.D., Ph.D.
- Michael Shoykhet, M.D., Ph.D.
Preterm birth is a major pediatric public health concern. Today, as many as 1 to 2 percent of all live births are preterm, with a survival rate of 85 to 90 percent. However, as many as 30- 50 percent of infants who survive preterm birth have cerebral palsy, intellectual disability, or other cognitive handicaps.
While some preterm infants progressively improve, a significant proportion still suffer major cognitive deficits, many have repeated a school grade by age 8, and more than 50 percent receive special education services at school. Circulatory disturbances and oxygen deprivation are the two major causes of neurodevelopmental impairments in these children. Hypoxia, due to lung immaturity and respiratory disturbances, is an important mechanism underlying these devastating neurological complications at this critical time in development. The research program on perinatal hypoxia and brain injury continues as a collaborative effort between Dr. Gallo’s research team (Drs. Jablonska, Forbes and Scafidi) and Flora Vaccarino, M.D., (Child Study Center, Yale University). Dr. Scafidi (supported by a K08 Award from the National Institute of Neurological Disorders and Stroke) and Dr. Jablonska study the developing brain by using a clinically relevant mouse model of chronic sublethal hypoxic injury. This model reproduces the brain injury hallmarks found in children, including cognitive behavioral abnormalities. Pre-clinical studies are combined with clinical research on premature babies and with postmortem human brain tissue.
Dr. Scafidi is a clinician scientist and his research is focused on understanding the endogenous repair mechanism of the brain after developmental brain injury. Using clinically relevant models of premature brain injury, he studies the effect of epidermal growth factor receptor signaling on recovery and whether pharmaceutical manipulation of these pathways promotes cellular and functional recovery. He uses multidisciplinary techniques to assess recovery, such as cellular and ultrastructural imaging, behavior, neuroimaging, and physiology.
Dr. Penn uses the mouse model of chronic sublethal hypoxic injury to study potential neuroprotective hormones that may improve neurodevelopmental outcomes when given before or after injury. The lab is investigating the potential of neurosteroids to reverse white matter damage in the cerebellum after developmental brain injury.
Drs. Gallo and Chew, together with Joseph Abbah, B.Pharm, Ph.D., (postdoctoral fellow) and Claire-Marie Vacher, Ph.D., (staff scientist), continue their studies of the cellular effects of hyperoxia on the developing brain, in particular on hippocampal development and function. In view of the effects of prematurity on learning and cognitive function, current studies focus on this brain region, which mediates memory formation and storage. Because of its role in continuous postnatal neurogenesis and remodeling/ synaptic plasticity, the hippocampus is particularly vulnerable to insults, leading to profound consequences in cognitive function. This team is focusing on defining specific hyperoxia-induced injury in selective hippocampal neuronal populations and the molecular pathways that mediate developmental abnormalities in these neurons.
Derangement of fetal circulatory flow due to congenital heart disease affects many vital organs; without proper supply of oxygenated blood, the brain is particularly vulnerable. Drs. Ishibashi and Jonas continue their studies of the cellular effects of hypoxia on gyrencephalic cortical and white matter development using a mouse and porcine developmental model. They found that preoperative hypoxia alters the neuroprotective function of astrocytes. Recovering this function before surgery may be a therapeutic option to reduce postoperative white matter injury in the immature brain.
Brain injury in cardiac arrest is regionally heterogeneous despite a global hypoxic-ischemic insult. What are the determinants of selective vulnerability or resistance to injury in the brain? Dr. Shoykhet’s research focuses on understanding how neurons and their projections in specific, behaviorally-relevant circuits are affected by hypoxia, ischemia, and reperfusion during development. Using the rodent somatosensory system, Dr. Shoykhet’s laboratory employs neurophysiologic recordings in animals, immunohistochemical analyses in stained tissue and behavior paradigms to study the impact of asphyxial cardiac arrest on thalamocortical circuitry and corresponding animal behavior. The long-term goal of the laboratory is to establish the mechanistic basis for selective vulnerability of defined neuronal population to hypoxic-ischemic insult and to employ this knowledge in designing targeted neuroprotective and rehabilitative strategies applicable to human survivors of cardiac arrest.
Mesenchymal stromal cells (MSCs) are multipotent, nonhematopoietic cells that possess both immunomodulatory and regenerative properties, and can treat a wide range of diseases including hypoxic brain injury. Multiple clinical trials have established the safety of MSC-based therapy. Using a translational preclinical model, Dr. Ishibashi is testing hypothesis that MSC delivery to the early postnatal brain promotes endogenous regeneration of damaged neuronal and glia cells in children with congenital heart disease (CHD). The team is proposing the use of cardiopulmonary bypass system itself as a new MSC delivery system in the CHD population.
Epilepsy
- Madison Berl, Ph.D.
- William D. Gaillard, M.D.
- Joan Conry, M.D.
- Tammy N. Tsuchida, M.D.
- Chandan Vaidya, Ph.D.
- Chima Oluigbo, M.D.
- Steven Weinstein, M.D.
- John Schreiber, M.D.
The lifetime risk of experiencing epilepsy is 1 in 27. Epilepsy has far-reaching consequences on brain structure and function, as well as significant morbidity and mortality. The Children’s National Pediatric Epilepsy Program (CPEP) continues to play a leading national and international role in the evaluation, care, and investigation of children with epilepsy. Co-morbidities of epilepsy (ADHD, anxiety, depression) adversely affect the quality of life in children with epilepsy. Dr. Gaillard along with Barbara Kroner, Ph.D. (RTI International), completed a Centers for Disease Control and Prevention study to investigate access to care and to identify co-morbidities in children with epilepsy who live in the District of Columbia. A seizure detection device shows promise for detecting generalized tonic-clonic seizures. With Dr. Berl and support from the BAND foundation the team surveyed physician practice for identification of co-morbidities and risks for sudden unexpected death in epilepsy. Dr. Sepeta investigates the development of memory systems. Her work suggests that children do not exhibit specialized localization episodic memory systems until adolescence, which may explain why children experience fewer memory deficits than adults who undergo temporal lobe epilepsy surgery.
CPEP also plays a central role in several national initiatives and repositories for neonatal seizures, neonates at risk to develop infantile spasms, pediatric status epilepticus, infantile epilepsy, and infantile spasms. Dr. Conry continues to lead industry-sponsored and federally-funded drug trials for children with epilepsy. Based on preliminary experience, Dr. Tsuchida is modifying a novel hybrid EEG electrode device for newborns (NEMO); she is also the lead neonatal neurologist for setting the standards for neonatal EEG through the American Clinical Neurophysiological Society. Dr. Schreiber with Dr. Lowell from cardiology have identified a potential marker of cardiac strain in children with refractory epilepsy. Dr. Oluigbo’s work has shown improved short-term epilepsy surgery outcomes using intraoperative MRI to optimize total resection of focal cortical dysplasia, the most common cause of intractable epilepsy and in pioneering minimally invasive techniques for epilepsy surgery.
Neuro-Oncology/Neurofibromatosis
- Maria Acosta, M.D.
- Kristina Hardy, Psy.D.
- Aerang Kim, M.D., Ph.D.
- Roger Packer, M.D.
- Joey Scafidi, M.D.
- Karen Walsh, Psy.D.
- Elizabeth Wells, M.D.
- Yuan Zhu, Ph.D. (Center for Cancer and Immunology Research)
Brain tumors are the most common solid cancers of childhood. Directed by Dr. Packer, the Children’s National Brain Tumor Institute continues to be a leading program with renewed National Cancer Institute (NCI) funding through the Pediatric Brain Tumor Consortium (PBTC). The Brain Tumor Institute has continued to develop new lines of investigation. A study of a novel inhibitor of mutant p53 has been opened with industry. The Brain Tumor Institute has become a member of the Pediatric (Pacific) Neuro-oncology Consortium and as such has opened a molecularly targeted treatment approach using tumor tissue removed at diagnosis by stereotactic biopsy to guide molecularly based therapy for brain stem gliomas. In addition, tissue removal at biopsy is being utilized to select patients for a post-radiotherapy vaccine trial. The tissue removed at diagnosis in children with brainstem gliomas is also being utilized by Dr. Nazarian (Center for Genetic Medicine) to develop novel molecular and immunologic approaches to treat this disease. Similar work is underway for children with medulloblastomas in the laboratory of Dr. Pei. Children’s National has banked more than 1,000 brain tumor specimens in our brain tumor laboratories and, in addition, has recently become a charter member of the national Children’s Brain Tumor Tissue Consortium under the leadership of Brian Rood, M.D.
Through the Pediatric Brain Tumor Consortium, novel immunotherapy approaches are being studied, including a study chaired nationally by Eugene Hwang, M.D., on the use of a checkpoint inhibitor. The institute is participating in a new, international effort, Defeat Pediatric GBM (glioblastoma multiforme), coordinated by the National Brain Tumor Society. This international consortium will have both a translational and a basic research component. Dr. Packer is the chair of this effort to link laboratories across the United States, as well as opening a personalized (precise) molecularly targeted therapeutic approach for children with newly diagnosed GBM.
The Neuro-oncology program has maintained its status as a full member of the Pediatric Brain Tumor Consortium in a competitive review. It has also been active in the Pediatric Neuro-oncology Consortium (PNOC) and the Sunshine Consortium. It has become a charter member of the novel agent brain tumor consortium, CONNECT. The program is focused on the development of the novel therapeutics including protocols focused on neuro-immunologic approaches.
The Gilbert Family Neurofibromatosis Institute is recognized as a center of excellence in clinical care and clinical research and is a pioneer in the biological development and implementation of interventions for oncology-related problems in neurofibromatosis 1 (NF1). The institute has launched multiple molecularly targeted investigations. Dr. Kim opened an international protocol evaluating the efficacy of a heat shock protein inhibitor for patients with NF-1 and malignant peripheral nerve sheath tumors. Dr. Packer remains group chair of the Department of Defense (DOD) Neurofibromatosis Clinical Trials Consortium, through which Children’s National has opened two molecularly targeted therapies for children with NF-1 and plexiform neurofibromas, utilizing drugs aimed at intracellular signaling (an MEK inhibitor disrupting RAS/MAPK signaling and a multi-kinase inhibitor). The institute continues its work on developing therapeutic approaches to prevent the devastating manifestations of NF-1, including neurocognitive sequelae and the development of visual pathway gliomas. The institute has continued to develop optical coherence tomography (OCT) as a predictive marker of impending visual loss in patients with NF-1 and visual pathway gliomas. Dr. Stephen Stasheff is adding to this effort by exploring the role of retinal injury in visual loss. Children’s National has opened an innovative study identifying the immuno-signature of neurofibromatosis associated gliomas.
Miriam Bornhorst, M.D., has joined the Neurofibromatosis Institute and has been awarded both a Hyundai and a Francis S. Collins Scholarship (through the Neurofibromatosis Therapeutic Acceleration Program) to continue her work on means to prevent the development of neurofibromatosis-related visual pathway gliomas. She also directs the Brain Tumor Cancer Genetics Program at Children’s National. Dr. Acosta’s work in the institute, which is funded by the DOD, continues to evaluate the efficacy of computerized-based cognitive rehabilitation/training and stimulant medication to improve neurocognitive function, including memory and executive functioning abilities in children with NF-1.
Dr. Zhu is continuing investigation on the utility of MEK inhibitors (MEKi) to prevent the development of a variety of NF1-associated diseases. Building on the observation of a therapeutic window for MEKi to prevent the formation of a developmental structural brain defect, his team has identified a similar therapeutic window during neonatal stages in which loss of NF-1 leads to defects in both neuronal and glial precursors during cerebellar development. Importantly, MEKi treatment during neonatal stages significantly rescues the developmental defects in NF1- deficient cerebellum, providing a long-term benefit for motor function. These studies provide strong preclinical evidence that a single MEKi agent administered during postnatal stages can prevent the formation of developmental structural brain defects, providing long-term benefits for brain structures and behaviors. Dr. Zhu’s group has also utilized a series of genetic systems to identify the therapeutic window of NF1-related optic pathway gliomas (OPG), which mainly occur in children younger than 7 years of age with NF-1.
Autism Spectrum Disorders
- Joshua Corbin, Ph.D.
- William D. Gaillard, M.D.
- Lauren Kenworthy, Ph.D.
- Cara Pugliese Ph.D.
- Chandan J. Vaidya, Ph.D.
- John Strang, Ph.D.
- Sinan Turnacioglu, M.D.
- Adelaide Robb, M.D. (Center for Translational Science)
The Center for Autism Spectrum Disorders (CASD), directed by Dr. Kenworthy, focuses on probing mechanisms of autism and developing and disseminating treatments and tools that drive successful transition into adulthood. CASD hosts 2 K awardees: Dr. Pugliese to investigate the efficacy and neural correlates of a school based executive function intervention for students with ASD who are college bound and Dr. Strang to understand behavioral, anthropometric and neural correlates in co-occurring autism and gender dysphoria, a critical but overlooked aspect of ASD. Dr. Kenworthy is principal investigator of a National Institute of Mental Health (NIMH) trial for an on-line training platform for parents of autistic children which targets executive function skills. She is also part of an RO1 with Georgetown University to investigate neural and cognitive profiles of executive function in developmental disabilities. Dr. Kenworthy and CASD via collaboration with Kevin Pelphrey, Ph.D., (director, Autism and Neurodevelopmental Disorders Institute, GW) have recently been awarded a prestigious NIMH Center of Excellence grant investigating gender related differences in autism and engaging autistic self-advocates to enrich and validate clinical and biological descriptions of autism through their lived experience of the disorder. Dr. Kenworthy, in collaboration with Dr. Corbin--with support from DC-IDDRC pilot funding--are exploring genotype to phenotype links in ASD bridging animal model work with humans. These efforts, along with continuing collaborations across multiple disciplines including human genetics, animal models, human imaging and behavioral assessments, are working to propel the understanding of ASD at multiple levels.
Selected Publications from 17/18
- Kay RB and Triplett JW. Visual neurons in the superior colliculus innervated by Islet2+ and Islet- retinal ganglion cells display distinct tuning properties. Frontiers in Neural Circuits 2017 Oct 10;11:73.
- Torii, M., Sasaki, M., Son, A.I., Mohammad, S., Chang, Y., Waxman, G.S., Kocsis, D. J., Rakic, P. and Hashimoto-Torii, K. Early detection of cellular damage by environmental and physical insults in mice Proc. Natl. Acad. Sci. U. S. A., 2017, 114(9):2367-2372
- Ishii, S., Torii, M., Son, A.I., Rajendraprasad, M., Morozov, M.Y., Imamura Kawasawa Y., Salzberg, A.C., Fujimoto, M., Brennand K., Nakai, A., Mezger, V., Gage, F., Rakic, P. and Hashimoto-Torii, K., Variations in brain defects result from cellular mosaicism in response to prenatal stress Nat. Commun., 2017 8:15157
- Morton PD, Korotcova L, Lewis BK, Bhuvanendran S, Ramachandra SD, Zurakowski D, Zhang J, Mori S, Frank JA, Jonas, RA, Gallo V, Ishibashi N.Abnormal neurogenesis and cortical growth in congenital heart disease. Sci Transl Med 2017 Jan 25;9(374). pii: eaah7029. (PMID: 28123074, PMCID: PMC5467873)
- Li P, Fu X, Smith NA, Ziobro J, Curiel J, Tenga MJ, Martin B, Freedman S, Cea-Del Rio CA, Oboti L, Tsuchida TN, Oluigbo C, Yaun A, Magge SN, O'Neill B, Kao A, Zelleke TG, Depositario-Cabacar DT, Ghimbovschi S, Knoblach S, Ho CY, Corbin JG, Goodkin HP, Vicini S, Huntsman MM, Gaillard WD, Valdez G, Liu JS. Loss of CLOCK Results in Dysfunction of Brain Circuits Underlying Focal Epilepsy. Neuron. 2017 Oct 11;96(2):387,401.e6.
- Stokes BA, Sabatino JA, Zohn IE. High levels of iron supplementation prevents neural tube defects in the Fpn1(ffe) mouse model. Birth Defects Res. 2017 Jan 30;109(2):81-91
- Lischinsky JE, Sokolowski K, Li P, Esumi S, Kamal Y, Goodrich M, Oboti L, Hammond TR, Krishnamoorthy M, Feldman D, Huntsman M, Liu J, Corbin JG. Embryonic transcription factor expression in mice predicts medial amygdala neuronal identity and sex-specific responses to innate behavioral cues. eLife. 2017;6.
- Adams KL, Gallo V. The diversity and disparity of the glial scar. Nat Neurosci. Epub 2017. Review.
- Andescavage NN, du Plessis A, McCarter R, Serag A, Evangelou I, Vezina G, Robertson R, Limperopoulos C. Complex Trajectories of Brain Development in the Healthy Human Fetus. Cereb Cortex. 2017 Nov 1;27(11):5274-83.
- Strang JF, Anthony LG, Yerys BE, Hardy KK, Wallace GL, Armour AC, Dudley K, Kenworthy L. The Flexibility Scale: Development and Preliminary Validation of a Cognitive Flexibility Measure in Children with Autism Spectrum Disorders. J Autism Dev Disord. 2017 Aug;47(8):2502-18.
- King AA, Seidel K, Di C, Leisenring WM, Perkins SM, Krull KR, Sklar CA, Green DM, Armstrong GT, Zeltzer LK, Wells E, Stovall M, Ullrich NJ, Oeffinger KC, Robison LL, Packer RJ. Long-term neurologic health and psychosocial function of adult survivors of childhood medulloblastoma/PNET: a report from the Childhood Cancer Survivor Study. Neuro Oncol. 2017 May 1;19(5):689-98.
- Sepeta LN, Casaletto KB, Terwilliger V, Facella-Ervolini J, Sady M, Mayo J, Gaillard WD, Berl MM. The role of executive functioning in memory performance in pediatric focal epilepsy. Epilepsia. 2017 Feb;58(2):300-10.