Children’s National Research Institute | Academic Annual Report 2017-2018
Innovation Through Collaboration
Institutes, Centers, & Offices
Center for Translational Science
Vision: Optimize health of children, families, and communities through the discovery, translation, and implementation of science. Mission: Catalyze interdisciplinary, collaborative investigation to accelerate discovery across the bench, bedside, and community continuum.
The Center for Translational Science (CTS) is broad based, non-categorical and includes a diverse portfolio of investigator-initiated research, participation in a wide range of national consortia, and providing key infrastructure resources. The center’s research activities are enhanced by the close partnership with the Clinical and Translational Science Institute at Children’s National (CTSI-CN), a partnership with The George Washington University (GW) funded by the National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program.
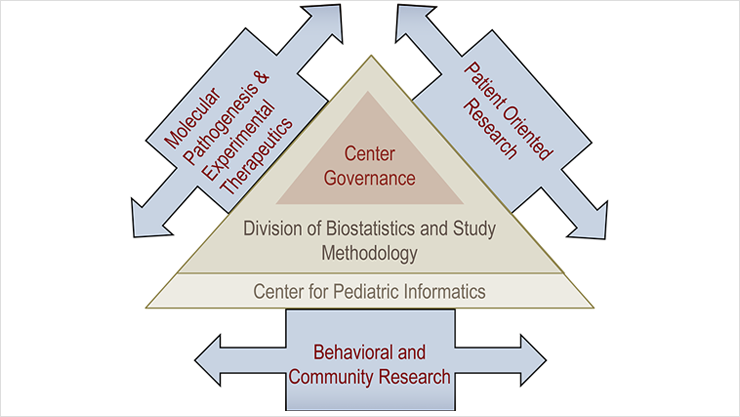
The center is organized into three major sub-themes that reflect the broad base of its investigator-initiated research (Figure 1): Molecular Pathogenesis and Experimental Therapeutics; Patient-Oriented Research; and Behavioral and Community Research. These sub-themes include investigator-initiated programs, as well as NIH-funded consortia, in which Children’s National Hospital (CNH) researchers play leadership roles. Within the Patient-Oriented Research sub-theme, reducing symptoms and preventing complications of illnesses are emphasized. Within the Behavioral and Community Research sub-theme, there is an emphasis on pediatric health services and health disparity research. Studies conducted by CTS faculty extend along the full spectrum of translational research (Figure 2).
Investigators are supported by three cross-disciplinary programs: the Division of Biostatistics and Study Methodology, the Center for Pediatric Informatics/Bioinformatics Unit, and the Office for Grants Enhancement. The latter, under the direction of Peter Scheidt, M.D., MPH, is a partnership with the CTSI-CN and provides critical support for junior faculty in writing and implementing career development awards, a mechanism for monitoring the progress of early-stage investigators, and a venue for review or critique of R-level NIH grant applications from established investigators.
In addition, the center has a portfolio of Special Interest Groups (SIGs) that serve as “catalyst programs” for specific research themes involving a broad range of investigators within the center and from the greater CRI community.
Leadership
-
Lisa M. Guay-Woodford, M.D.
Director; Richard L. and Agnes F. Hudson Professor of Health Services Research; Director, Clinical and Translational Science Institute at Children’s National (CTSI-CN; a CTSA-funded partnership with The George Washington University)
Executive Committee
-
John van den Anker, M.D., Ph.D.
Evan and Cindy Jones Chair in Pediatric Clinical Pharmacology; Vice Chair of Pediatrics for Experimental Therapeutics; Chief, Division of Clinical Pharmacology -
Adelaide Robb, M.D.
Professor of Pediatrics; Chief, Division of Psychiatry and Behavioral Medicine -
Randi Streisand, Ph.D., CDE
Professor, Psychology & Behavioral Health; Interim Chief of Psychology and Behavioral Health -
Pamela S. Hinds, Ph.D., RN, FAAN
William and Joanne Conway Chair in Nursing Research; Executive Director of Nursing Science, Professional Practice, and Quality Outcomes
Faculty
- Claude Abdallah, M.D., M.Sc., Anesthesiology and Pain Medicine
- Nicholas Ah Mew, M.D., Genetics and Metabolism
- Shireen Atabaki, M.D., MPH, Emergency Medicine
- Laura Ball, Ph.D., Speech and Hearing
- Mark L. Batshaw, M.D., Developmental Pediatrics; Chief Academic Officer and Physician-in-Chief
- Nancy Bauman, M.D., Otolaryngology
- Andrea Beaton, M.D., Cardiology
- Lee Beers, M.D., Goldberg Center for Community Pediatric Health
- John Berger, M.D., Director, Cardiac Intensive Care Unit
- James E. Bost, Ph.D., Biostatistics and Study Methodology
- Kathleen Brown, M.D., Emergency Medicine
- Randall Burd, M.D., Ph.D., Trauma and Burn Services
- James Chamberlain, M.D., Emergency Medicine
- Hollis Chaney, M.D., Pulmonary Medicine
- Kimberly Chapman, M.D., Ph.D., Fetal Medicine Institute and Rare Disease Institute – Genetics and Metabolism
- Irene Chatoor, M.D., Psychiatry
- Kevin Cleary, Ph.D., Bioengineering Initiative
- Shayna Coburn, Ph.D., Psychology
- Laurie Conklin, M.D., Gastroenterology, Hepatology and Nutrition
- Denice E. Cora-Bramble, M.D., MBA, Ambulatory and Community Health Services
- Michele Dadson, Ph.D., Psychology
- Deepika Darbari, M.D., Hematology
- Nathan Dean, M.D., Critical Care Medicine
- Roberta DeBiasi, M.D., Infectious Diseases
- Emmanuele C. Delot, Ph.D.
- Nina Deutsch, M.D., Anesthesiology and Pain Medicine
- Katherine Deye, M.D., Child and Adolescent Protection Center
- Daniel Fagbuyi, M.D., Emergency Medicine
- Karen Fratantoni, M.D., MPH, Community Pediatric Health
- Linda Yu-Sing Fu, M.D., M.Sc., Community Pediatric Health
- Leandra Godoy, Ph.D., Infant and Toddler Mental Health
- Heather Gordish-Dressman, Ph.D., Biostatistics and Study Methodology
- Monika Goyal, M.D., MSCE, Emergency Medicine
- Michael Guerrera, M.D., Hematology
- Ellie Hamburger, M.D., FAAP
- Rana Hamdy, M.D., MPH, MSCE, Infectious Disease
- Raafat S. Hannallah, M.D., Anesthesiology and Pain Medicine
- Nada Harik, M.D., Infectious Disease
- Linda Herbert, Ph.D., Psychology
- Stacy Hodgkinson, Ph.D., Psychology
- Brian Jacobs, M.D., Critical Care Medicine
- Marni Jacobs, Ph.D., Biostatistics and Study Methodology
- Barbara Jantausch, M.D., Infectious Disease
- Anitha John, M.D., Cardiology
- Yewande Johnson, M.D., Anesthesiology and Pain Medicine
- Paul Kaplowitz, M.D., Ph.D., Endocrinology and Diabetes
- Katherine Kelly, Ph.D., RN, Nursing Research and Quality Outcomes
- Benny Kerzner, M.D., Gastroenterology
- DongKyu Kim, Ph.D., Biostatistics and Study Methodology
- Terry Kind, M.D., MPH, Community Pediatric Health
- Anastassios Koumbourlis, M.D., Pulmonary Medicine
- Naomi Luban, M.D., Hematology and Laboratory Medicine Services
- Maureen E. Lyon, Ph.D., ABPP, Psychology
- Eleanor Mackey, Ph.D., Psychology
- Darlene Mansoor, M.D., Allergy and Immunology
- Kalyani Marathe, M.D., Dermatology
- Gerard Martin, M.D., Cardiology
- Robert McCarter, Sc.D., Biostatistics and Study Methodology
- William McClintock, M.D., Neurology
- Chaya Merrill, Dr.Ph., Child Health Advocacy Institute
- Michele Mietus-Snyder, M.D., Cardiology
- Nazrat M. Mirza, M.D., Sc.D., Community Pediatric Health
- Jeffrey Moak, M.D., Community Pediatric Health
- Maureen Monaghan, Ph.D., Psychology
- Asha Moudgil, M.D., Nephrology
- Nickie Niforatos, M.D., Neonatology
- Karen O’Connell, M.D., Emergency Medicine
- Tessie W. October, M.D., MPH, Critical Care Medicine
- Mary Ottolini, M.D., MPH, Hospitalist Medicine
- Kavita Parikh, M.D., Hospitalist Medicine
- Sophie Pestieau, M.D., Anesthesiology and Pain Medicine
- Murray Pollack, M.D., Critical Care Medicine
- Khodayar Rais-Bahrami, M.D., Neonatology
- Natella Rakhmanina, M.D., Ph.D., Infectious Disease, HIV Medicine
- Craig Sable, M.D., Cardiology
- Peter Scheidt, M.D., Grants and Enhancement Program
- Hemant Sharma, M.D., MHS, Allergy and Immunology
- William Sheehan, M.D., Allergy and Immunology
- Lamia Soghier, M.D., Neonatology
- Xiaoyan Song, Ph.D., MBBS, M.Sc., Infectious Disease
- Pranoot Tanpaiboon, M.D., Genetics and Metabolism
- Anupama Tate, D.M.D., Oral Health
- Stephen J. Teach, M.D., MPH, Emergency Medicine
- Lisa Tuchman, M.D., MPH, Adolescent and Young Medicine
- Carrie Tully, Ph.D., Psychiatry and Behavioral Sciences
- Janelle Vaughns, M.D., Anesthesiology, Sedation, and Perioperative Medicine
- Susan Thomas Verghese, M.D., Anesthesiology and Pain Medicine
- Jichuan Wang, Ph.D., Biostatistics and Study Methodology
- Yunfei Wang, M.D., Biostatistics and Study Methodology
- David Wessel, M.D., Critical Care Medicine
- Edward Wong, M.D., Laboratory Medicine Services
- Angela Wratney, M.D., MHSc, FAAP, Critical Care Medicine
- Pavel Yarmolenko, Ph.D.
Center Research Programs
NIH-Funded Consortia
Hepato-Renal Fibrocystic Disease Core Center
- Lisa M. Guay-Woodford, M.D.
Funded through an NIH P30 mechanism, Dr. Guay-Woodford founded the Hepato-Renal Fibrocystic Disease Translational Core Center (HFRDCC) in 2005 during her tenure at the University of Alabama at Birmingham. Autosomal recessive polycystic kidney disease (ARPKD) and other hepato-renal fibrocystic diseases (HRFDs) are relatively rare genetic disorders, but they constitute an important set of childhood nephropathies. Rare-disease research requires greater collaboration than in common diseases to permit the creation of larger databases and repositories.
Within the HRFDCC, Dr. Guay-Woodford established the Hepato-Renal Fibrocystic Diseases Translational Resource (Core A) that features a longitudinal clinical database, a human tissue repository, a DNA Bank, and a database for genetic mutations causing HRFDs, drawn from tertiary care centers throughout the Americas. Core A also has developed a portfolio of educational information and tools that highlights ARPKD but also encompasses the spectrum of HRFDs. Through the P30 mechanism, this core resource serves as a critical platform for assessing genotype-phenotype correlations, identifying new HRFD genes, and developing future interventional studies. In addition, this core provides educational resources to the broad community of patients and families and physicians/health care providers.
Inner City Asthma Consortium
- Stephen J. Teach, M.D., MPH
- Dinesh Pillai, M.D. (Division of Pulmonary and Sleep Medicine)
- William Sheehan, M.D. (Division of Allergy and Immunology)
With support from the National Institute of Allergy and Infectious Diseases (NIAID), the Inner City Asthma Consortium (ICAC) consists of 10 national sites and provides infrastructure for investigator-initiated studies of multiple clinical and translational aspects of immuno-monitoring and immuno-therapy among urban, disadvantaged and largely minority children with moderate to severe asthma and atopy. Led at Children’s National Hospital by Dr. Teach and now in its 14th year of continuous funding, the ICAC has recently inaugurated major prospective studies of the role of anti-IL-5 in mitigating morbidity in eosinophilic asthma and of desensitization in cockroach sensitive children.
Pediatric Clinical Pharmacology Research Program
- John van den Anker, M.D., Ph.D.
- Janelle Vaughns, M.D.
- Elaine Williams, Ph.D.
- Natella Rakhmanina, M.D., Ph.D.
- Andrea Hahn, M.D.
- Shogo Miyagi, Pharm.D., Ph.D.
- Mae Liu, Pharm.D.
The Pediatric Clinical Pharmacology Research Program is funded by the National Institute of Child Health and Human Development (NICHD) Research Center in Pediatric Developmental Pharmacology (2011-2016 and 2016-2021) and is one of only four such centers across the nation. Each of these centers is specifically dedicated to support translational science in the area of pediatric clinical pharmacology. In addition, Children’s National was awarded by NICHD a T32 grant for postdoctoral training in Pediatric Clinical Pharmacology, one of only five such T32s in the nation. This new T32 award allows collaborations with Johns Hopkins University School of Medicine, the U.S. Food and Drug Administration (FDA) and the School of Pharmacy at the University of Maryland. Over the years the program has supported several investigators, including Drs. Chamberlain, Rakhmanina, Robb and Vaughns, in securing NIH and other external funding. Data from each of these studies promises to improve the safe and effective use of medicines for newborn infants, children and adolescents with HIV, seizures, psychiatric disorders, obesity, and pain management.
Pediatric Emergency Care Applied Research Network
- James M. Chamberlain, M.D.
- Stephen J. Teach, M.D., MPH
- Shireen M. Atabaki, M.D., MPH
- Kathleen M. Brown, M.D.
- Karen O’Connell, M.D., M.Ed.
- Monika Goyal, M.D., M.S.C.E.
The federally-funded Health Resource Service Administration (HRSA)/Maternal and Child Health Bureau (MCHB)/Emergency Medical Services for Children (EMSC) network is led by six national principal investigators, including Dr. Chamberlain, and supports a host of clinical and translational effects dedicated to improving care and outcomes for acutely ill and injured children. In the past year, the Pediatric Emergency Care Applied Research Network (PECARN) completed a randomized controlled trial of approximately 1,200 patients with diabetic ketoacidosis to determine optimal fluid management. A study of audit and feedback for more than 400 providers using the electronic health record at seven sites was also completed. PECARN initiated a study of biosignatures to distinguish bacterial from viral infections, and continued patient enrollment in a 45-site randomized controlled trial with the Neurologic Emergencies Treatment Trials (NETT) network to define the optimal drug treatment for children with prolonged seizures who have failed initial therapy with benzodiazepines.
Rare Diseases Clinical Research Center – The Urea Cycle Disorders Consortium (UCDC)
- Andrea Gropman, M.D.
- Mendel Tuchman, M.D.
- Mark Batshaw, M.D.
- Nicholas Ah Mew, M.D.
- Ljubica Caldovic, Ph.D.
- Hiroki Morizono, Ph.D.
- Dashuang Shi, Ph.D.
The Urea Cycle Disorders Consortium (UCDC) is an NIH-funded (since 2003) 16-site research consortium within the Rare Disease Clinical Research Network formed to investigate inborn errors of the urea cycle. These rare genetic disorders result from defects in any of the eight genes associated with this important metabolic cycle and have a combined prevalence of about 1 in 30,000. Urea cycle disorders (UCDs) lead to the accumulation of ammonia in the blood and brain and resultant episodes of metabolic encephalopathy, with a great risk of morbidity and mortality. The focus of the UCDC is to perform a longitudinal natural history study and intervention studies of these disorders and to develop and test new diagnostic and therapeutic approaches. Children’s National serves as the leadership hub of the consortium, which is led by Dr. Gropman. The UCDC is supported by funding from the NIH and the Kettering and O’Malley Family Foundations. In the past decade, the consortium has successfully brought to market three new drugs to treat hyperammonemia and currently follows more than 700 individuals with these disorders.
The Collaborative Pediatric Critical Care Research Network
- Murray Pollack, M.D.
- David Wessel, M.D.
- Randall Burd, M.D.
Since 2005, NIH has funded the Collaborative Pediatric Critical Care Research Network (CPCCRN) to investigate the safety and efficacy of treatments, management strategies, and outcomes of critically ill children in intensive care units. Children’s National has been a member of this network since its inception. The network currently consists of seven clinical sites and a data-coordinating center. Led at Children’s National by Drs. Wessel (co-principal investigator), Pollack (co-principal investigator), and Burd (co-investigator), CPCCRN has published over 50 peer-reviewed manuscripts from numerous observational trials on diverse subjects, including cortisol response in critical illness, near-fatal asthma, critical pertussis, assessment of morbidity, predicting morbidity and mortality, a decision support tool for mechanical ventilation, bleeding and thrombotic complications of extracorporeal membrane oxygenation (ECMO), and opioid tolerance.
The program is currently engaged in a broad range of studies, including, evaluation of correlations between hemodynamics during CPR with outcomes, implementation of a resuscitation bundle to improve cardiac arrest outcomes, GMCSF (granulocyte-macrophage colony-stimulating factor) to improve immune function following critical illness, uses of inhaled nitric oxide, microbiome changes preceding ventilator associated pneumonia, red blood cell changes during sepsis, life after pediatric sepsis, hypothermia impact on pharmacology, phenotypes of sepsis, causes and needed new therapies for PICU morbidity and mortality, and mediators of pediatric ARDS (adult respiratory distress syndrome). In collaboration with Pediatric Emergency Care Applied Research Network (PECARN) and the National Heart, Lung, and Blood Institute (NHLBI), CPCCRN is conducting a randomized trial of therapeutic hypothermia after pediatric cardiac arrest (THAPCA).
Patient-Oriented Research
Improving Pediatric Asthma Care in the District of Columbia (IMPACT DC)
- Stephen J. Teach, M.D., MPH
- Randi Streisand, Ph.D.
- Shilpa Patel, M.D.
- Will Sheehan, M.D.
- Dinesh Pillai, M.D.
Focusing on the epidemic of asthma among the disadvantaged and mostly minority children in the District of Columbia, Dr. Teach leads a multidisciplinary and highly collaborative program spanning the full spectrum of clinical and translational research. The program, known as IMPACT DC, for “Improving Pediatric Asthma Care in the District of Columbia,” has funding from NIAID, NHLBI, Patient-Centered Outcomes Research Institute (PCORI), the Department of Health of the District of Columbia and several foundations. The program works to address the disparities in care and outcomes among inner-city children with asthma in the District, while serving as a model program for the nation. IMPACT DC’s research effects and collaborations include elements of T1-T4 translational research.
As a site principal investigator with the NIAID-funded Inner City Asthma Consortium and with the infrastructural support of the CTSI-CN at Children’s National, Dr. Teach has studied novel immunomonitoring and immunotherapy in asthma. With support from the NHLBI as part of a large multicenter effort, the team is studying the role of the poly-antigenic oral bacterial extract given to infants in preventing the onset of later topic wheeze. At the other end of the translational spectrum, Dr. Teach collaborates with Dr. Streisand (Division of Psychology & Behavioral Health) on a PCORI-funded, randomized clinical trial of psychosocial stress management for parents of at-risk urban youth with asthma in an effort to improve their children’s asthma care and outcomes.
Congenital Heart Disease Newborn Screening Program
- Gerard R. Martin, M.D.
- Lisa A. Hom, RN, Esq
The team continues to provide leadership in research, advocacy, education and the implementation of newborn screening for critical congenital heart disease (CCHD). Since the 2011 publication of best practices for implementing CCHD screening in pediatrics, screening is now required in 48 states and the District of Columbia. With a focus on collaboration and advocacy, the team has worked with families, the American Academy of Pediatrics (AAP), the March of Dimes, the American College of Cardiology, and the American Heart Association on the passage of the Healthy Hearts of Babies Act of 2015. This legislation, which took effect in September 2015, requires CCHD screening in the District of Columbia.
Pediatric Psychopharmacology Team
- Michele Dadson, Ph.D.
- Julia Dorfman, M.D., Ph.D.
- Lisa Efron, Ph.D.
- Adelaide Robb, M.D.
This program and its team from the Division of Psychology and Behavioral Health, is funded by both industry and the NIH to investigate new therapies for psychiatric and behavioral disorders in children ages 5 to 17 years. The Team collaborates with investigators across the U.S. and around the world to identify best practices in testing psychotropic medications for both safety and efficacy. Areas of interest span the psychiatric field from autism to schizophrenia. In addition, the team is a training resource for other investigators on best practices for evaluating study subjects, consenting practices and retention of enrolled participants. Trials with frequent pharmacokinetic sampling often incorporate the expertise of the CTSI-CN nursing and ancillary staff. Recent studies have included a phase I trial of a novel antidepressant where Children’s National was the first center to establish dosing in a pediatric cohort. This agent is currently undergoing phase III study at multiple sites including Children’s National. A second national trial is evaluating add-on treatment for aggression in ADHD, using a hand held device to record aggressive behavior which is then loaded directly into the study database, optimizing data accuracy and eliminating parental recall bias. Since its inception the Psychopharmacology Trials Team has conducted over 75 registration trials leading to the FDA approval of multiple medications in attention deficit hyperactivity disorders (ADHD), autism, bipolar disorder, major depression, obsessive compulsive disorder and schizophrenia.
Improving Pediatric Trauma Resuscitation
- Randall Burd, M.D., Ph.D.
This program focuses on improving teamwork during trauma resuscitation and improving pre-hospital pediatric trauma triage. Dr. Burd leads a multidisciplinary research team that studies errors and teamwork in trauma resuscitation, with collaborators in emergency medicine and surgery, informatics, computer science, and biomedical engineering. In addition, he directs R01-funded projects to develop statistical approaches for real-time prediction of outcome after pediatric injury and to build an approach for automatic tracking and monitoring of teamwork during trauma resuscitation.
Management of Severe Infections in Special Populations
- Roberta DeBiasi, M.D., M.S.
This program involves multiple clinical trials focused on evaluation and treatment of severe viral infections that affect pregnant women, neonates, immunocompromised hosts and typically-developing children. With funding from the NIAID/NIH Collaborative Antiviral Study Group, Dr. DeBiasi is evaluating: 1) maternal herpes simplex virus shedding at delivery using rapid diagnostics; 2) the natural history of neonatal herpes simplex virus infection; pharmacokinetics and pharmacodynamics of antiviral therapy in premature infants with congenital cytomegalovirus infection; and 3) antiviral treatment of sensorineural hearing loss following congenital cytomegalovirus infection. The broader portfolio focuses on a range of infectious disease issues, including 1) novel antiviral and plasma treatments for severe hospitalized influenza and parainfluenza infection; 2) congenital Zika infection with particular emphasis on prenatal imaging, testing, genetics and virological features; 3) Ebola response and preparedness; and 4) the burden of pediatric Lyme disease as well as the long-term outcomes of Lyme infection in children. Dr. DeBiasi is also the site’s principal investigator (PI) for a PCORI-funded multicenter clinical trial evaluating optimal management of refractory Kawasaki Disease.
Prevention and Early Detection of Rheumatic Heart Disease
- Andrea Beaton, M.D.
- Craig Sable, M.D.
Drs. Beaton and Sable work within a large international collaborative group on research projects aimed at reducing the global burden of rheumatic heart disease (RHD). Dr. Beaton, a former KL2 scholar, is currently funded through the American Heart Association Strategically Focused Research Network. With Dr. Sable as the program director, Dr. Beaton is the clinical project PI working towards a better understanding of acute rheumatic fever in endemic countries. Further, she has recently received support from the Thrasher Research Fund to conduct a clinical trial in Uganda to determine the value of secondary prophylaxis to prevent the progression of early rheumatic heart disease. In addition, foundation funding supports work examining the impact of handheld echocardiography on decentralization of cardiac care in low-resource settings (Uganda), improving the feasibility of echocardiographic screening for RHD through task-shifting and use of ultra-portable devices (Uganda), and on understanding the epidemiology of Group A streptococcal infections in sub-Saharan Africa. Additionally, a new grant from the Edwards Lifesciences Foundation has launched a research study in Brazil to examine the ability to integrate screening for heart valve disease into the primary health care structure of the country.
Hearing and Speech Research
- Laura J. Ball, Ph.D., C.C.C./S.L.P.
The Hearing and Speech Research program has a broad research portfolio that includes: 1) instrument development and validation; 2) characterization of speech, swallowing, and functional communication of children with rare diseases; 3) development of novel technologies and strategies for augmentative and alternative communication (AAC); 4) improving patient-provider communication for children with severe communication disorders using AAC visual representations; 5) evaluating disparities in language development following cochlear implantation; and 6) pediatric swallowing disorder characterization, assessment, and outcomes post assessment. Dr. Ball is a co-investigator on the NIH-funded Stanford-Harvard collaborative group that studies the application of a brain-computer communication interface for those locked-in with paralysis. She has established collaborations with Children’s National investigators (Divisions of Neurological Sciences and Physical Medicine and Rehabilitation); GW (Departments of Speech-Language Pathology and Audiology, Anatomy and Regenerative Biology), Children’s Hospital of Philadelphia (Division of Neurology), and University of South Dakota (Department of Communication Sciences and Disorders).
Measuring Child-Reported Symptom, Functional Status and Treatment Toxicity Experiences
- Pamela S. Hinds, Ph.D., RN., F.A.A.N.
- Jichuan Wang, Ph.D.
- Shana Jacobs, M.D.
- Catriona Mowbray, Ph.D.
- Emily Stern, BSN
Children being treated for acute and chronic illnesses can experience symptoms from both the illness itself as well as from its treatment. If the child is not asked to report the functional consequences of the illness and its treatment, the full disease impact remains unknown and the symptoms may be undertreated. The team has continued to lead the assessment of the pediatric Patient Reported Outcomes Measurement Information System (PROMIS) in children receiving treatment for various cancers, as well as in survivors of childhood cancer. Outcomes include the acceptability of the PROMIS measures to children and adolescents, the ability of children with advanced and incurable cancer to complete the forms, the different forms of validity achieved by these measures in this patient group, and the highly similar measurement outcomes from the pediatric PROMIS measures as compared to previously well-validated and established pediatric instruments for child reporting of symptoms and function. With collaborative funding awarded in 2015, the team is pursuing next studies to address the clinical usefulness of these pediatric PROMIS measures. In addition, the team continues to co-lead an eight site study to create and validate a child-reported cancer treatment toxicity measure. Outcomes include final lists of core and expanded treatment toxicities validated by children 7 to 18 years of age, development of parallel parent report toxicity measures and validated directions and response options for both sets of measures and construct validity from 275 children, parents and their clinicians.
Pediatric Critical Care Outcomes Research Initiative (PC-CORI)
- Murray M Pollack, M.D.
The overarching goal of this initiative is to develop a dynamic assessment of risk suitable for use in individual hospitalized patients. The effort is focused on using big data from the Health Facts® database with over 50 million patient encounters. The initiative combines the clinical expertise of critical care medicine, the biostatical expertise of Children’s National Division of Biostatistics and Study Methodology, the data sciences expertise of GW Biomedical Informatics Center, and computer and data management expertise of the CTSI – CN and GW. Other goals of the initiative include the investigation of medication use and medication complications, the identification of previously undefined clinical states important for outcome prediction, and the long-term goal of making big data available to the Children’s National and GW community. The initiative is funded from support of the Mallinckrodt Pharmaceuticals.
Behavioral and Community Research
Improving Care of Youth with Type 1 Diabetes
- Randi Streisand, Ph.D., C.D.E.
- Maureen Monaghan, Ph.D.
Families of children diagnosed with type 1 diabetes confront daunting tasks every day, such as administering insulin injections, monitoring blood glucose levels, and paying careful attention to diet and physical activity. While adhering to a complex diabetes regimen, parents also try to ensure normative activities and opportunities throughout childhood into young adulthood. NIH funds Drs. Streisand and Monaghan to identify new mechanisms to support youth and families and to optimize diabetes management. Dr. Streisand is specifically investigating two behavioral interventions aimed at parents of very young children with diabetes. Dr. Monaghan is evaluating health behaviors that contribute to successful independent self-management and transition to adult medical care for young adults with diabetes, and is funded by the American Diabetes Association to evaluate an intervention to promote positive communication between young adults and their health care provider. Drs. Streisand and Monaghan’s comprehensive research program is designed to improve family care, reduce parent and child stress, and ultimately promote improved health outcomes across the lifespan for youth with diabetes.
Food Allergy Management and Adjustment among Youth
- Linda Hebert, Ph.D.
More than 40 percent of U.S. children with food allergy experience severe allergic reactions. The majority of fatal food allergic reactions occur during adolescence and young adulthood, indicating that adolescence is a period of risk for reduced allergen avoidance and epinephrine carriage. Food allergy also leads to anxiety regarding allergen exposure, and at least one-third of children with allergies experience food allergy-related bullying. With K23 funding from NIAID, Dr. Hebert and her team are focused on determining how to facilitate healthy adolescent adjustment to food allergy. The team is collecting comprehensive medical and psychosocial data from up to 150 adolescents with food allergy and their parents at three time points over the course of one year. The goal of the project is to develop a model of factors related to food allergy anxiety, quality of life, and adherence (allergen avoidance, epinephrine carriage) that will directly lead to development of clinical interventions for this population.
Transition from Pediatric to Adult Care for Adolescents with Complex Chronic Conditions
- Lisa Tuchman, M.D., MPH
Transition from pediatric to adult care for adolescents with severe chronic disorders is a major health care challenge, which might interfere with quality of care of these patients. Dr. Tuchman and her team draw upon clinical and advocacy experience in caring for chronically ill adolescents and young adults by focusing research effects on improving the health care transition process from pediatric to adult- oriented care. Research aims to improve the quality, safety, efficiency and effectiveness of the delivery of chronic care management in the setting of health care transition. Dr. Tuchman is PI on a Maternal and Child Health Bureau-funded grant aimed to address unmet mental health needs amount transition aged youth cared for at Children’s National. She also serves as co-investigator on multiple federally-funded projects designed to improve care transitions and self-management skills for chronically ill adolescents, including those with cystic fibrosis, hemophilia, and sickle cell disease and survivors of childhood cancer. This research contributes to the development of evidence-based transition programs in clinical settings nationwide.
The Role of Parent Navigators in Successful Transition of NICU Graduates
- Karen Fratantoni, M.D., MPH
- Lisa Tuchman, M.D., MPH
- Randi Streisand, Ph.D., C.D.E.
Drs. Fratantoni, Tuchman and Streisand are funded by a PCORI award to study how parent navigators can help families and children with fragile medical conditions successfully manage the transition from the Neonatal Intensive Care Unit (NICU) to home. No previous studies have evaluated the effectiveness of long-term peer support on the ability of families transitioning from the NICU to achieve self-efficacy and infant health. The project will assess the impact of the Children’s Parent Navigator Program in the NICU, expanding the role of the existing Parent Navigator Program in the Children’s National Diana L. and Stephen A. Goldberg Center for Community Pediatric Health that currently provides a medical home to children with complex special health care needs.
Improving Parent Clinician Communication During Critical Illness
- Tessie October, M.D., MPH
Dr. October and her team are funded by a NIH K23 award to evaluate strategies for improving parent clinician communication during decision-making for critically ill children. The team is pilot testing a communication skills training intervention targeted to clinicians and assessing this intervention in terms of outcomes at the parent, patient, and clinician level. As a young investigator, Dr. October has received additional funding from the Children’s National Hospital Board of Visitors grant, a CTSI-CN Voucher Award, and The George Washington University Fellowship program to support her research program.
Improving Hospital-to-Home Transition for Children
- Kavita Parikh, M.D., MSHS
Dr. Parikh and her team are funded by a career development award from the Agency of Health Research and Quality. She is piloting a community-based hospital-to-home transition plan for patients and their caregivers after a hospitalization for an asthma exacerbation. A patient-centered transition plan is being developed from qualitative interviews with stakeholders, including caregivers, asthma educators, primary care physicians, hospitalists, pulmonologists, school nurses and payers. The program will be piloted by enrolling children during a hospitalization for an asthma exacerbation and following them outpatient.
Addressing the Needs of Children and Young Adults with Life-Limiting Conditions
- Maureen E. Lyon, Ph.D., ABPP
- Jichuan Wang, Ph.D.
Dr. Lyon is funded by the National Institute of Nursing Research (NINR) to study advance care planning in teens with cancer, and teens and adults with HIV/AIDS. The adult-focused work is in collaboration with the NIH-funded District of Columbia Center for AIDS Research (DC-CFAR). This research program includes a multidisciplinary team of 41 investigators at 13 study sites, including physicians, nurses, psychologists, social workers, clinical coordinators and graduate students. These collaborative teams have focused on palliative care for HIV-positive persons in Appalachia and geographic mapping of palliative care use among severely ill children. A new collaboration with the University of Minnesota involves a pilot study of the Family-centered Advance CarE (FACE) planning intervention developed for teens about to undergo a bone marrow transplant. The team is also exploring the impact of advance care planning on treatment adherence.
Community-Based Mental Health and Family Support
- Lee Savio Beers, M.D.
- Leandra Godoy, Ph.D.
- Melissa Long, M.D.
Dr. Beers is the director of the DC Collaborative for Mental Health in Pediatric Primary Care and the DC Mental Health Access in Pediatrics (DC MAP) Program. Both initiatives are designed to improve the integration of mental health into primary care to improve access and quality. Research and evaluation focus on interventions designed to increase access to care. For example, recent evaluation of a longitudinal, quality improvement learning collaborative demonstrated significant increases in routine mental health screening at pediatric well-visits. Other initiatives include a pilot study evaluating the impact of integrating Certified Family and Peer Support specialists into the DC MAP program, in order to improve family engagement in mental health services, and analysis of patterns of mental health screening and referral in primary care.
In partnership with MedStar Georgetown University Hospital, Dr. Beers co-directs the Early Childhood Innovation Network (ECIN), a transformative and innovative approach to reducing the impact of adversity and community deprivation on young children in the District of Columbia. ECIN provides a platform for intervention evaluation as well as systems-based research, with a focus on rapid cycle evaluation.
Nursing Research
- Pamela S. Hinds, Ph.D., RN, FAAN
- Katherine Patterson Kelly, Ph.D.
- Mia Waldron, MSN-ED, RN-BC, CPN
- Vicki Freedenberg, Ph.D., RN
- Nadine Camp, DNP, APRN, CPNP-PC
The Department of Nursing Science, Professional Practice, and Quality supports a collection of more than 20 clinical studies and eight quality improvement science projects led by nurse investigators. Studies include behavioral interventions, instrumentation testing, evaluation of nursing care procedures, treatment communication and decision-making, and systematic assessments of child and family responses to illness threat from diagnosis to health recovery or to end of life. Example study outcomes in the past year include 1) the validation of a theory related to child preference for involvement in treatment communication and decision making; 2) the acceptability and feasibility of a primary palliative care program implemented in more than 18 clinical care areas; 3) expanding from four units to nine units the implementation of a safety measure that allows registered nurses to nap on the nightshift; 4) the acceptability and feasibility of hospitalized children and their parents to give their satisfaction ratings with care prior to discharge from the hospital stay; and 5) the implementation of care guidelines for non-pharmacological treatment of pain during intrusive procedures. NIH grants support the exploration of the internal definition of “being a good parent to my seriously ill child” and the link to parent health and family well-being before and following a child’s death, the validation of a child-reported common treatment toxicity measure, and sophisticated analyses of child-reported treatment toxicities.
Improving Disparities in Health and Health Care
Children’s National has a long-standing commitment to ameliorating disparities in health and health care that affect the many disadvantaged, low-income, and minority children in the Washington metro area. Collectively, these projects reinforce the ongoing engagement of Children’s National in the local community through collaborative research that applies rigorous scientific inquiry to better understand and effectively address health disparities.
DC Baltimore Center for Research on Child Health Disparities
- Nazrat Mirza, M.D., Sc.D.
- Randi Streisand, Ph.D., CDE
- Stacy Hodgkinson, Ph.D.
- Leandra Godoy, Ph.D.
Dr. Mirza serves as the Children’s National PI for this NIH P20-funded program, which supports work to prevent type 2 diabetes in young adults, and to promote quality of life and well-being in teens affected by violence. The center brings together investigators in the Children’s National Hospital Goldberg Center for Community Pediatric Health, as well as Howard University, and Johns Hopkins University. Dr. Hodgkinson organizes the active Community Advisory Board, and the Research Core (Drs. Mirza, Streisand and Godoy) provides mentoring for junior faculty, and supports four new child health disparities research pilot studies by junior faculty.
HIV-AIDS
- Natella Rakhmanina, M.D., Ph.D.
Dr. Rakhmanina and her team, in partnership with the NIH-funded DC-CFAR, are studying a longitudinal cohort of HIV-infected and HIV-exposed children and adolescents. In addition, the team conducts pharmacologic studies of the antiretroviral drugs in women, children and adolescents. Dr. Rakhmanina serves as a senior technical advisor at the Elizabeth Glaser Pediatric AIDS Foundation working on multiple international pediatric and adolescent HIV projects in several African countries. She also serves as a member of the U.S. Department of Health and Human Services Panel on the Pediatric Antiretroviral Therapy and Management Guidelines at the Office of AIDS Research Advisory Council in the NIH and as a member of the Pediatric AIDS Committee at American Academy of Pediatrics.
Bioenergetics Group
- Michele Mietus-Snyder, M.D.
- Nazrat Mirza, M.D., Sc.D.
- Evan Nadler, M.D.
- Eleanor Mackey, Ph.D.
- Laura Fischer, Ph.D., RD
- Robert Freishtat, M.D.
- Rachel Walega, MS
- Whitney Osborne, MPH
The Bioenergetics Special Interest Group has a focus on collaborative research around obesity, weight reduction strategies, and cardio-metabolic risk. Drs. Nadler, Mackey, and Mietus-Snyder have collaborated to explore the cognitive impact of severe obesity and changes with weight loss following bariatric surgery. Dr. Mietus-Snyder is funded through the NHLBI Pediatric Heart Network as site-PI in a multi-site randomized controlled trial studying the impact of statins in dyslipidemia, the most prevalent manifestation of cardiometabolic risk, the Dyslipidemia of Obesity Intervention in Teens (DO IT) project. In addition, the community outreach initiated by Dr. Mietus-Snyder in 2012, Kid Power (KiPOWTM) continues to accelerate and support D.C. public and public charter school wellness policy in partnership with medical student health mentors from GW. As participating medical students move on to residency programs, Dr. Mietus-Snyder has helped them to establish new KiPOW sites, e.g. Children’s Hospital Orange County (CHOC) and University of Texas Southwestern in Dallas. Comparable and encouraging results from CHOC, an institution with greater than 95 percent Hispanic student population and Children’s National, who works with a greater than 99 percent African American student population, are an important validation of this mentored behavioral change model.
Achieving Health Equity in Emergency Department Care
- Monika Goyal, M.D., MSCE
Dr. Goyal is a pediatric emergency medicine physician, a health services researcher and epidemiologist, who leads a research program designed to reduce racial and ethnic disparities in the provision of health care. Her NIH-funded research, focuses on improving the delivery of sexual and reproductive health services in the emergency department (ED). Furthermore, this program seeks to understand racial and ethnic disparities in the provision of ED care for children with the ultimate goal to sustainable interventions that provide equitable care to all children accessing ED care.
Immunization Delivery
- Linda Fu, M.D.
- Jichuan Wang, Ph.D.
Understanding and removing barriers to children receiving recommended vaccinations requires a multi-pronged approach. Dr. Fu’s research, funded by a recent NIH K23 award, examines factors that impact vaccination coverage at the personal socio-behavioral, community, health care provider and health system levels. Studies were completed recently on the social influences that impact human papillomavirus (HPV) vaccine acceptance among African-American parents. With the increasing number of recommended adolescent immunizations such as HPV vaccine, college student health centers are a logical setting in which to focus improvement initiatives. The team is examining adolescent vaccination coverage rates among a national sample of college students to determine the impact of a virtual quality improvement learning collaborative on improving college students' immunization coverage. In a new collaborative project with Pfizer, Drs. Fu and Wang will use respondent-driven sampling techniques to determine early childhood immunization coverage rates and barriers to immunization among the District of Columbia’s homeless children, a population that is likely under-represented in national immunization surveys.
Centralized Support of Clinical and Translational Research
Over the past decade, Children’s National has experienced a marked growth of research, which, in large part, is attributable to NIH grants that provide centralized support for research (such as cores), and multicenter consortia in which novel, rigorous research can be conducted. Such grants account for approximately 20 percent of all CRI funding, support the career development of many junior faculty members, and facilitate the work of a diverse spectrum of investigators. The Center for Translational Science has developed key support in areas such as biostatistics, multicenter clinical trials, grants development and, more recently, informatics. These infrastructural resources work in close partnership with the CTSI-CN. Key components of the collaborative center infrastructure include the following:
Division of Biostatistics and Study Methodology (Partnership with the CTSI-CN)
- James E. Bost, Ph.D.
- Robert McCarter, Sc.D.
- Heather Gordish-Dressman, Ph.D.
- Marni Jacobs, Ph.D.
- Dongkyu Kim, Ph.D.
- Jichuan Wang, Ph.D.
- Yunfei Wang, Ph.D.
The Division of Biostatistics and Study Methodology (BSM) is led by Dr. Bost. The division includes six additional faculty members and eight staff. Prior to joining Children’s National, Dr. Bost was the director of the Outcomes Center at Children’s Healthcare of Atlanta.
As experts in the implementation and analysis of trials, registries, observational studies and quality improvement initiatives, the BSM team tailors statistical analyses and data management to meet the specific needs of investigators in study design, including data analysis plans, and sample size considerations. At study implementation, the division provides operations and regulatory support, including monitoring visits, randomization implementation, electronic web-based data capture (EDC) systems, as well as data management support. At study conclusion, the division statistical data analyses and results interpretation to address research questions.
The division collaborates with investigators from all CRI centers and investigators from the hospital’s clinical divisions, as well as partners at GW (through the CTSI-CN), Cincinnati Children’s Medical Center and the University of Pittsburgh. In addition to housing the Biostatistics, Epidemiology, and Research Design (BERD) component of the CTSI-CN, the division is involved in several external networks, such as the Cooperative International Neuromuscular Research Group (CINRG, Center for Genetic Medicine Research) and the RDCRN Urea Cycle Disorders (UCD) Consortium.
Center for Pediatric Informatics/Bioinformatics Unit (Partnership with the CTSI-CN)
- Hiroki Morizono, Ph.D.
- Brian Jacobs, M.D.
- DongKyu Kim, Ph.D.
- Mohammed Khan, MS
The Center for Pediatric Informatics at Children’s National was established in 2006 as a multi-disciplinary group comprising faculty and staff with informatics and technology background and/or interest to optimally develop and use the electronic health record (EHR) to both understand and improve the quality of health care delivery, research and education for children. The center’s primary goals have been to utilize novel information technology, computer science, and knowledge management methods to deliver safer and more effective care, increase the efficiency of care delivery, improve disease prevention, increase the effectiveness of translational research, improve knowledge access and technology-enhanced education, and enhance regulatory compliance.
In 2013, Children’s National formed a strategic partnership with the Cerner Corporation to create the Bear Institute, the first pediatric health IT institute. The Bear Institute focuses on operational excellence and innovation, in both clinical care and hypothesis-driven investigation. These efforts embrace and closely collaborate with the faculty and staff and initiatives in the Center for Pediatric Informatics.
Over the past year, the Center for Pediatric Informatics has transitioned into the CRI Bioinformatics Unit, sponsored by a partnership that includes the Center for Translational Science, the Center for Genetic Medicine Research, the CTSI-CN, the DC-IDDRC and the office of the CRI Chief Research Officer. This new unit is envisioned as a cross-center academic home for informaticians and computational biologists, and an administrative home that provides CRI investigators with informatics support for next generation sequencing and other ‘omics analyses, as well as converting EHR data into useful information for research focused on clinical effectiveness, quality improvement, and evidence-based care.
Grants Enhancement Program (Partnership with the CTSI-CN)
- Peter Scheidt, M.D., MPH
- Stephan Ladisch, M.D.
- Mendel Tuchman, M.D.
- Cynthia Rand, Ph.D. (Johns Hopkins University)
- Christina Robinson, MA
The Grants Enhancement Program (GEP), established under CTSI-CN, is co-led by Drs. Scheidt and Ladisch. The GEP provides support for junior faculty seeking grant funding. The program’s goal is to improve grant applications submitted by Children National’s junior faculty and new investigators in order to maximize the likelihood of success. It supports and guides junior faculty as well as mid- and senior-level faculty when requested in the development of competitive proposals to obtain funding. Internal review, feedback, and consultation on proposals provided by the program faculty (in addition to those of mentors and supervisors) are the most important functions of this resource. Reviews and consultations are available and conducted at all stages in the course of developing a proposal, from the initial draft of specific aims to a final application. When appropriate subject-matter expertise is not available at Children’s National and the proposal is considered well-developed and competitive, the program facilitates and assists with obtaining in-depth external review by a carefully selected, experienced external reviewer.
The program also organizes and leads monthly group meetings with peer investigators who are at the same level as those seeking Mentored Career Development Awards (the K Group) and for those seeking R01 type funding (the R Group). Through these group activities, participants share current information on the entire process of grant preparation, access examples of successful applications and other supporting materials and obtain feedback and critique from their peers on their own evolving proposals. The program provides a detailed checklist for the final assembly of NIH grant applications and other guidance for seeking extramural funding.
Since its inception, the GEP has received 261 protocols for review at various stages of development. Of the 210 proposals submitted for funding that have been reviewed, 82 (39 percent) were funded.
Special Interest Groups in the Center for Translational Science
The Center for Translational Science actively supports the work of six interdisciplinary special interest groups (SIGs), organized as collaborative hubs for defined areas of research focus within the center. The SIGs generate new research initiatives and connect these to clinical care priorities. These SIGs and their facilitators include:
- Bioenergetics SIG (Lead: Dr. Mietus-Snyder)
- Behavioral and Community Research SIG (Lead: Dr. Streisand)
- Pediatric Palliative and End-of-Life SIG (Leads: Drs. Hinds, Lyon, and LaFond)
- Sexual and Reproductive Health SIG (Leads: Drs. Goyal and L. Tuchman)
- Global Health SIG (Leads: Drs. Rakhmanina and Beaton)
- Child Health Disparities SIG (Lead: Dr. Godoy)
- Informatics and Big Data SIG (Lead: Dr. Morizono)
SIG research targets include the rising prevalence of obesity and associated cardiometabolic risks in socioeconomically disadvantaged children; treatment compliance in adolescents with diabetes; soliciting and honoring child and parent preferences for end-of-life care, large-scale screening and treating of adolescents with sexually transmitted diseases and global health issues, particularly those related to infectious diseases, health disparities, and innovative technologies used with large datasets. Active membership in each of the SIGs ranges from 10 to 25 investigators, with more than 10 disciplines represented. In the past academic year, the SIGs hosted 25 scientific presentations and one international plenary, submitted four grants, and published multiple collaborative papers.
Selected Publications from 17/18
- Alzarka B, Morizono H, Bollman JW, Kim D, Guay-Woodford LM. Design and Implementation of the Hepatorenal Fibrocystic Disease Core Center Clinical Database: A Centralized Resource for Characterizing Autosomal Recessive Polycystic Kidney Disease and Other Hepatorenal Fibrocystic Diseases. Front Pediatr. 2017 Apr 20;5:80.
- Beaton A, Aliku T, Dewyer A, Jacobs M, Jiang J, Longenecker CT, Lubega S, McCarter R, Mirabel M, Mirembe G, Namuyonga J, Okello E, Scheel A, Tenywa E, Sable C, Lwabi P. Latent Rheumatic Heart Disease: Identifying the Children at Highest Risk of Unfavorable Outcome. Circulation. 2017 Dec 5;136(23):2233-44.
- Beers LS, Godoy L, Biel MG. Using Effective Public Private Collaboration to Advance Integrated Care. Child Adolesc Psychiatr Clin N Am. 2017 Oct;26(4):665-75.
- Berger JT, Holubkov R, Reeder R, Wessel DL, Meert K, Berg RA, Bell MJ, Tamburro R, Dean JM, Pollack MM, Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network. Morbidity and mortality prediction in pediatric heart surgery: Physiological profiles and surgical complexity. J Thorac Cardiovasc Surg. 2017 Aug;154(2):620,628.e6.
- Cook S, Stockmann C, Samiee-Zafarghandy S, King A, Deutsch N, Williams E, Wilkins D, Sherwin C, van den Anker J. Neonatal maturation of paracetamol (acetaminophen) glucuronidation, sulphation, and oxidation based on a parent-metabolite population pharmacokinetic model. Clin Pharmacokinet 2016;55(11):1395-1411.
- Dallas RH, Kimmel A, Wilkins ML, Rana S, Garcia A, Cheng YI, Wang J, Lyon ME, Adolescent Palliative Care Consortium. Acceptability of Family-Centered Advanced Care Planning for Adolescents With HIV. Pediatrics. 2016 Dec;138(6):e20161854.
- Findling RL, Robb AS, DelBello M, Huss M, McNamara N, Sarkis E, Scheffer R, Poulsen LH, Chen G, Lemming OM, Areberg J, Auby P. Pharmacokinetics and Safety of Vortioxetine in Pediatric Patients. J Child Adolesc Psychopharmacol. 2017 Aug;27(6):526-34.
- Goyal MK, Fein JA, Badolato GM, Shea JA, Trent ME, Teach SJ, Zaoutis TE, Chamberlain JM. A Computerized Sexual Health Survey Improves Testing for Sexually Transmitted Infection in a Pediatric Emergency Department. J Pediatr. 2017 Apr;183:147,152.e1.
- Hilliard ME, Tully C, Monaghan M, Wang J, Streisand R. Design and development of a stepped-care behavioral intervention to support parents of young children newly diagnosed with type 1 diabetes. Contemp Clin Trials. 2017 Nov;62:1-10.
- Hinds PS, Wang J, Stern. ED, Macpherson CF, Wharton CM, Okorosobo R, Cheng YI, Gross HE, Meany HJ, Jacobs S. Voices of children and adolescents on phase 1 or phase 2 cancer trials: A new trial endpoint? Cancer. 2017 Oct 1;123(19):3799-806.
- Natukunda E, Gaur AH, Kosalaraksa P, Batra J, Rakhmanina N, Porter D, Shao Y, Zhang H, Pikora C, Rhee MS. Safety, efficacy, and pharmacokinetics of single-tablet elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide in virologically suppressed, HIV-infected children: a single-arm, open-label trial. Lancet Child Adolesc Health. 2017;1(1):27-34.
- Pearce AL, Mackey E, Cherry JBC, Olson A, You X, Magge SN, Mietus-Snyder M, Nadler EP, Vaidya CJ. Effect of Adolescent Bariatric Surgery on the Brain and Cognition: A Pilot Study. Obesity (Silver Spring). 2017 Nov;25(11):1852-60.