The consortium started an annual competition in 2012 to fund companies and research labs that propose the best solutions to the unmet pediatric needs for medical devices. The program is already making promising headway in bringing more pediatric devices to market. Cases in point: NCC-PDI has helped startup companies to develop devices to diagnose pediatric ear infections and to draw blood non-invasively in inpatient settings. Children’s National is working with both companies to test and validate these devices.
Diagnosing otitis media more accurately
Each year, about one in 10 children in the U.S. develop otitis media, making it one of the most prevalent childhood diseases. For more than a century, physicians have primarily diagnosed otitis media the same way, with an otoscope—a tool akin to a penlight coupled with a magnifying glass. However, this method has relatively limited diagnostic power, with reported sensitivities and specificities ranging from just 50 percent to 75 percent among expert physicians.
This year, Children’s National clinicians including otolaryngologists Diego Preciado, M.D., Ph.D., and Nancy Bauman, M.D., collaborated with Champaign, Ill.-based medical device developer PhotoniCare to test a new tool for diagnosing otitis media.
The device, known as CLEARVIEW™, uses a light-based technology called optical coherence tomography (OCT). A mainstay technology for examining the retina in ophthalmology, OCT can peer through the tympanic membrane to image the middle ear, the site of otitis media infections. It can help clinicians detect the presence of biofilms and purulent effusions, signs of an ongoing infection.
After receiving direct funding support from NCC-PDI in 2015, PhotoniCare joined with clinicians and researchers at Children’s National, working together to develop a study protocol and obtain institutional review board approval to conduct a study testing this device in patients. The researchers then submitted a Phase II SBIR application that was funded for over $2 million by the National Institutes of Health’s National Institute on Deafness and Other Communication Disorders. A follow-on Phase II-b study will begin soon.
This device, the researchers say, is already showing promise toward helping physicians make more accurate diagnoses that can stem the use of unnecessary antibiotics or surgeries and help patients who need these interventions receive them sooner.
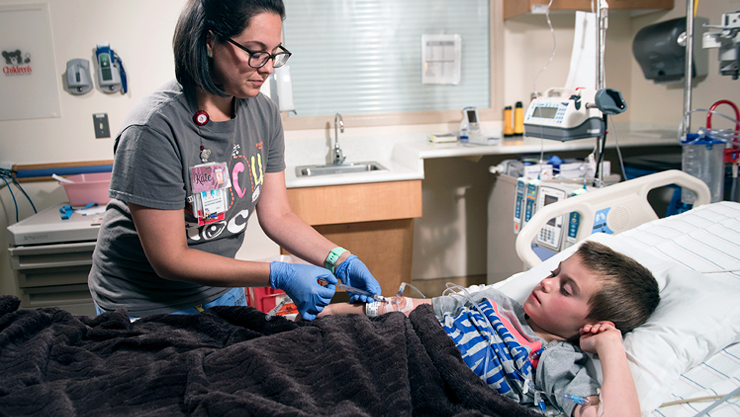
Blood draws without needles
More than 400 million blood draws take place each year at U.S. hospitals, offering a window into health information that can’t be acquired any other way. However, nearly half of these draws fail on the first try, requiring multiple sticks to get it right. For a pediatric patient, these sticks can be painful and traumatic—the reason why more than half of children experience high stress before, during and after needle sticks.
However, San Francisco based VelanoVascular is providing an alternative through a system called PIVO™ that allows needleless blood draws through the same peripheral intravenous catheter that many patients receive during hospital stays. Through NCC-PDI support and mentorship, the company was able to partner with Children’s National to study the feasibility of using this system in pediatric patients.
For years, VelanoVascular worked on developing a rigid tube that can be inserted into the catheter just for blood draws, doing away with the need for additional needle sticks once a patient’s IV tubing is already in place. This system has received U.S. Food and Drug Administration clearance and is being used in a number of large health systems. However, the gauges of the rigid tube currently available aren’t suitable for many hospitals’ youngest patients. The company is currently working with Children’s National clinicians and researchers, including Director of Interventional Radiology Karun Sharma, M.D., to develop smaller gauge devices that work with pediatric patients’ unique anatomy.
The team performed a prospective single-center study at Children’s National of the venous anatomy of the lower arm to describe valve frequencies, locations of branches or collateral vessels and vessel diameters central and peripheral to a placed peripheral IV. The study’s results suggested that the majority of adult and pediatric veins can accommodate 20- and 22-gauge peripheral IV catheters, respectively. A second study, which used ultrasound to evaluate venous changes associated with peripheral IV placement in infants, will inform design changes to the next-generation of PIVO™ with a 24-gauge size for infants.