Children’s National Research Institute | Academic Annual Report 2017-2018
Innovation Through Collaboration
The participant and clinical interactions module team
Clinical and Translational Science Institute at Children’s National (CTSI-CN)
Vision: Help every child reach their full potential and live a productive life through advances in clinical and translational research.
The Clinical and Translational Science Institute at Children’s National (CTSI-CN) was established in 2010 as a collaboration between Children’s National Hospital (CNH) and its academic partner, The George Washington University (GW) with funding from the Clinical and Translational Science Award (CTSA) program of the National Center for Advancing Translational Sciences (NCATS) at the National Institutes of Health (NIH). The CTSI-CN is one of 64 CTSA-funded institutions across the U.S. and has the distinction of being the only child-health focused program. The strategic mission of this program is to promote: 1) high-quality research, 2) efficient translation of discoveries to human applications, 3) effective implementation into clinical practice, and 4) improved quality of life for children and their families locally and across the country.
Leadership
-
Lisa Guay-Woodford, M.D.
Principal Investigator -
Robert Miller, Ph.D.
Co-Principal Investigator (The George Washington University)
Executive Committee
-
Lisa M. Guay-Woodford, M.D.
CNHS,Director -
Robert Miller, Ph.D.
GW, Co-Director -
Reamer Bushardt, Pharm.D.
CNHS, Lead, Translational Endeavors Core -
James Bost, Ph.D.
CNHS, Lead, Research Methods Core -
Adelaide Robb, M.D.
CNHS, Lead, Hub Liaison Team for Trial Innovation Network -
Naomi Luban, M.D.
CNHS, Program Lead, KL2 -
Karen McDonnell, Ph.D.
GW, Lead, Evaluation and Continuous Improvement Module (ex officio)
Faculty
- Karen McDonnell, Ph.D. (The George Washington University) and Robert McCarter, Sc.D., Co-Leads, Evaluation and Continuous Improvement
- Jesse Pines, M.D., MBA, FAAEM, (The George Washington University) and Asha Payne, M.D., Co-Leads, Quality and Efficiency
- Keith Crandall, Ph.D. (The George Washington University), Hiroki Morizono, Ph.D., Brian Jacobs, M.D., and Qing Zeng, Ph.D. (The George Washington University), Co-Leads, Informatics
- Kathleen Roche, MSW, Ph.D. (The George Washington University) and Chaya Merrill, DrPH, Co-Leads, Community Engagement
- Kevin Cleary, Ph.D., Sean Cleary, Ph.D., MPH (The George Washington University), Susan Knoblach, Ph.D., Gaetano Lotrecchiano, Ed.D., Ph.D. (The George Washington University), Co-Leads, Collaboration and Multidisciplinary Team Science
- Mary Ottolini, M.D., MPH, and Reamer Bushardt, PharmD, PA-C, DFAAPA (The George Washington University), Co-Leads, Translational Workforce Development
- Robert J. Freishtat, M.D., MPH and Tim McCaffrey, Ph.D. (The George Washington University), Co-Leads, Pilot Translational and Clinical Studies
- Peter Scheidt, M.D., Director, Grants Enhancement Program
- James Bost, Ph.D. and Samuel Simmens, Ph.D. (The George Washington University), Co-Leads, Biostatistics, Epidemiology and Research Design
- Lawrence Deyton, MPSH, M.D. (The George Washington University) and Thomas Silber, M.D., Co-Leads, Regulatory Knowledge and Support
- Catherine Limperopoulos, Ph.D. and Freya Spielberg, M.D., MPH (The George Washington University), Co-Leads, Integrating Special Populations
- Sheela Magge, M.D., MSCE, Gary Simon, M.D. (The George Washington University), and Melissa Napolitano, Ph.D. (The George Washington University), Co-Leads, Participant and Clinical Interactions
- Adelaide Robb, M.D. and Gary Simon, M.D. (The George Washington University), Co-Leads, Liaison to Trial Innovation Centers
- Olga Price, Ph.D. (The George Washington University), Madison Berl, Ph.D., and Dongkyu Kim, Ph.D., Co-Leads, Liaison to Recruitment Innovation Centers
- Peter Kim, M.D., Igor Efimov, Ph.D. (The George Washington University), John van den Anker, M.D., Ph.D., and Susan Knoblach, Ph.D., Co-Leads, Orphan Product Accelerator – Innovations Incubator
- Lisa M. Guay-Woodford, M.D., Lead, Child Health Research Through Multisite Planning (CHAMP)
CTSI-CN Framework
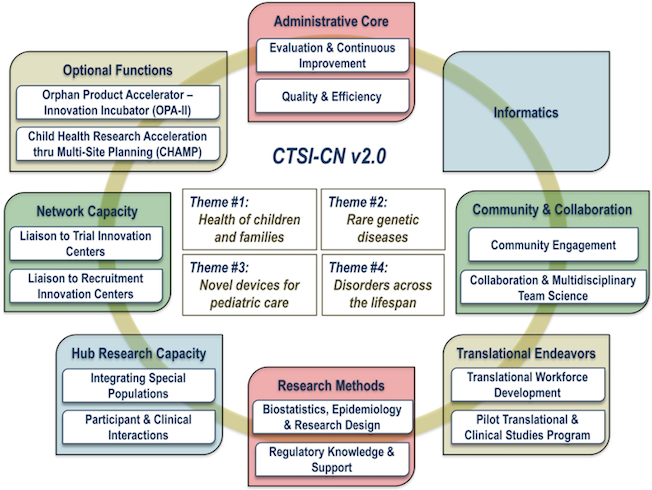
Cores’ functions and organizing themes
In July 2016, the CTSI-CN v2.0 was funded through a second, five-year, $24 million award from NCATS. CTSI-CN v2.0 provides highly integrated, cost-effective and investigator-focused resources in five key strategic areas that have been emphasized by NCATS to support effective clinical and translational research (CTR). These include:
- Workforce Development: Develop a new generation of diverse, high quality, child health-focused CTR investigators through innovative educational, training and mentoring programs.
- Collaboration and Engagement: Create innovative approaches and technologies to catalyze child-health CTR, through cross-disciplinary collaborations that engage multiple stakeholder communities.
- Integration: Foster multi-disciplinary teams capable of conducting child-health CTR across the translational spectrum from bench to communities to address health disparities, fetal/maternal medicine and rare genetic diseases.
- Methods and Processes: Catalyze the development/adaptation of study methodologies and streamline processes to enhance child-health focused CTR from conception to adulthood.
- Informatics: Advance child-health CTR through a comprehensive, integrated information ecosystem, with user-friendly training in informatics methods and tools.
CTSI-CN v2.0 is organized operationally into a set of modules (Figure 1) that are designed to: 1) support and empower basic, translational and clinical investigators to collaborate with one another and with community partners; 2) ease the administrative burdens of conducting research; and 3) break down traditional research barriers.
As examples, the Biostatistics, Epidemiology, and Research Design (BERD) team provides high quality biostatistical and epidemiological expertise for the development and design of pediatric and lifespan CTR. The Regulatory Knowledge and Support (RKS) module assists CTR investigators and their teams through proactive, innovative regulatory and research ethics education and support services to assure that child health CTR research meets the highest standards of ethical conduct and regulatory compliance. The Participant and Clinical Interactions (PCI) / Clinical Research Unit provides a high-quality, safe, and welcoming environment for pediatric study participants and investigators. PCI encompasses a variety of resources and services divided into sub-components, each providing specialized services for investigators and their clinical research protocols.
At the institutional level, the CTSI-CN has catalyzed new, child health research-focused interactions among faculty at Children’s National and GW. For example, The Trial Innovation Network (TIN) Hub Liaison Team serves as a resource for investigators to request consultations and services for multi-center clinical trials. Through the efforts of the Hub Liaison Team, both Children’s National and GW have agreed to use a single IRB system (SMART IRB) and streamline contracting workflows through the master Accelerated Clinical Trial Agreement (ACTA) and Accelerated Confidential Disclosure Agreement (ACDA). ACTA and ACDA facilitate relationships with industry sponsors that are interested in expediting the contract process, thus optimizing lag time for research.
In sum, the CTSI-CN is acknowledged locally as the hub for effective, high quality child health CTR and as a nurturing, integrated home for CTR team science. Nationally, the CTSI-CN has provided leadership in child health CTR, in both CTSA- and non-CTSA-linked consortia and steering committees.
Participation in CTSA Consortia
The CTSA Accrual to Clinical Trials (ACT)
- Lisa M. Guay-Woodford, M.D.
- Hiroki Morizono, Ph.D.
- Dongkyu Kim, Ph.D.
- Marcin Gierdalski, Ph.D.
The CTSA ACT is an NCATS-funded consortium that is focused on developing a nationwide, federated network of sites that share electronic health record (EHR) data with common standards, data terminology and resources. This network is designed to accelerate participant recruitment in clinical trials, which is a major limiting factor in translating discoveries into clinical applications. The ACT enables investigators to “float” queries across the consortium to rapidly identify cohorts of eligible participants in a de-identified way. Under informed consent, potential participants are then contacted to discuss participation in a specific trial and arrange for study-specific screening and enrollment. Within the network, each site designates a local investigator to serve as an “honest broker” who can obtain the necessary IRB consent and ensure the requisite oversights to protect participant privacy. Within the next year, the CTSI-CN will deploy access to the ACT network to thelocal investigator community.
CTSA Supplement
CNHS/NIAID Joint Program on Pediatric Disorders of Immunological Regulation
- Lisa M. Guay-Woodford, M.D.
- Michael Keller, M.D.
- Hemant Sharma, M.D.
- Roberta Debiasi, M.D.
- Maureen Monaghan, Ph.D.
Building on the infrastructure established by the CTSI-CN, the nationally recognized clinical excellence of Children’s National, and established collaborations between Children’s National and the National Institute of Allergy and Infectious Diseases (NIAID), the CTSI-CN is spearheading the implementation of an innovative, collaborative program to jointly address important problems facing children with disorders of immunological regulation. Funded as a CTSA Administrative Supplement in September 2017, the Program is designed to: 1) develop and implement joint research protocols for studying children with infectious, immunologic and allergic diseases, so as to advance the prevention, diagnosis, treatment, and cure of these disorders; 2) provide NIAID pediatric patients enrolled on higher risk protocols a greater level of pediatric specialty support and safety by moving their care from the NIH Clinical Center to CNHS, where there is an exclusive focus on pediatric care; and 3) provide joint training opportunities for investigators studying and caring for children with infectious, immunological, and allergic diseases.
The initial focus areas for this collaborative program are in infectious diseases, immunodeficiency diseases and food allergy. These investigative initiatives are complemented by clinical and research training in infectious diseases, allergy and immunology. The ability to expand and enhance training opportunities at each facility is consistent with the mission of the CTSI-CN and the national CTSA Network.
Selected Publications from 17/18
- Chahrour M, Kleiman RJ, Manzini MC. (2017). Translating genetic and preclinical findings into autism therapies. Dialogues Clin Neurosci. 19(4):335-343.
- Clark BC, Sumihara K, McCarter R, Berul CI and Moak JP. (2016). Getting to zero: impact of electroanatomical mapping on fluoroscopy use in pediatric catheter ablation. J Intervent Cardiac Electrophysiol 46:183-189.
- Dallas RH, Kimmel A, Wilkins ML, Rana S, Garcia A, Cheng YI, Wang J, Lyon ME. (2016). A Randomized Controlled Trial of FAmily-CEntered (FACE) Advanced Care Planning for Adolescents with HIV/AIDS: Emotions, Acceptability, Feasibility. Pediatrics Dec;138(6).
- Hahn A, Warnken S, Perez-Losada M, Freishtat RJ, Crandall KA. (2017). Microbial diversity within the airway microbiome in chronic pediatric lung diseases. Infect Genet Evol Dec 7. pii: S1567-1348(17)30432-X.
- Jaimes III, R., Swiercz, A., Sherman, M., Muselimyan, N., Marvar, P. J., and Posnack, N. G. (2017). Plastics and cardiovascular health: phthalates may disrupt heart rate variability and cardiovascular reactivity. Am J Physiol-Heart Circul Physiol 313: H1044-H1053.
- Hathout Y, Conklin LS, Seol H, Gordish-Dressman H, Brown KJ, Morgenroth LP, Nagaraju K, Heier CR, Damsker JM, van den Anker JN, Henricson E, Clemens PR, Mah JK, McDonald C, Hoffman EP. (2016). Serum pharmacodynamic biomarkers for chronic corticosteroid treatment of children. Sci Rep. Aug 17;6:31727.
- Mansoor A, Cerrolaza JJ, Perez G, Biggs E, Nino G, Linguraru MG. (2017). Marginal shape deep learning: Applications to pediatric lung field segmentation. Proc SPIE Int Soc Opt Eng. 2017;10133:10.1117/12.2254412.
- Osborn DPS, Pond HL, Mazaheri N, et al. (2017). Mutations in INPP5K cause a form of congenital muscular dystrophy overlapping Marinesco-Sjogren syndrome and dystroglycanopathy. Am J Hum Genet. 2017;100(3):537-545.
- Son AI, Opfermann JD, McCue C, et al. (2017). An implantable micro-caged device for direct local delivery of agents. Sci Rep. 7(1):17624-017-17912-y.
- Simms, M., Baumann, K., and Monaghan, M. (2017). Health communication experiences of emerging adults with Type 1 diabetes. Clinical Pract Pediatric Psychology 5(4), 415.
- Wang, J., Hefetz, A & Liberman, G. (2017). Applying structural equation modelling in educational research. Culture and Education. 29(3): 563–618.
- Webman RB, Carter EA, Mittal S, Wang J, Sathya C, Nathens AB, Nance ML, Madigan D, Burd RS. (2016). The relationship between center type and mortality among adolescent trauma patients. JAMA Pediatr Aug 1;170(8):780-786.