Children’s National Research Institute | Academic Annual Report 2017-2018
Innovation Through Collaboration
Sheikh Zayed Institute for Pediatric Surgical Innovation
Vision: Reimagining pediatric surgery, the Sheikh Zayed Institute for Pediatric Surgical Innovation at Children’s National Hospital combines research and clinical expertise into one collaborative program. The institute cultivates knowledge and develops products and procedures to benefit children in the Washington metro area, across the country and around the world.
A mission to “make pediatric surgery more precise, less invasive, and pain free” drives investigators at the Sheikh Zayed Institute (SZI). The team believes that innovation without manufacturing is not a real innovation. Through shared innovation and a spirit of collaboration, the team and its thought leaders spanning many industries (nonprofit, academia, corporate, advocacy and health care) join forces to successfully support pediatric product development for children everywhere. The institute stimulates meaningful engagement among all stakeholders—patients and families, clinicians, researchers, engineers, business professionals, industry partners and policymakers—as it works to improve children’s health through the following:
Path to Bedside. To close the gap between innovations and the commercially viable technologies that enter the market, the institute rolled out the Entrepreneur in Residence (EIR) Program to ensure that the innovators receive the resources needed to transform technology from a concept into a market-ready product. A number of the SZI Startups attracted commercial investments this year.
Efficiency and Accountability. To hold programs accountable to milestones, deliverables, and Go/No-Go time points, this effort ensures that promising programs receive the support they need to reach their goals. Programs that fall short of their targets are redirected or closed. All faculties are now funded from peer-reviewed national funding agencies. A significant amount of National Institutes of Health (NIH) funding for knowledge creation and translation was awarded to institute investigators, including Phase II SBIR grants.
Open Innovation Support. The Sheikh Zayed Institute is one of the seven FDA-funded sites in the United States to support pediatric device development through the total product development cycle. From regulatory to payer analysis consultation, the institute provides competitive funding and non-financial support. The team, supported by in-house regulatory consultants, engineers, and the Entrepreneur-In- Residence (EIR) Program facilitates both in- and outside investigators and entrepreneurs for pediatric-unmet-need-focused device development.
Faculty
Leadership
-
Anthony Sandler, M.D.
Director, Sheikh Zayed Institute; Senior Vice President and Surgeon-in-Chief -
Kolaleh Eskandanian, PhD, MBA, PMP
Vice President and Chief Innovation Officer
Senior Leadership
- Kevin Cleary, PhD
- Julia Finkel, MD
- Diego Preciado, MD, PhD
- Karun Sharma, MD, PhD
Faculty
- Shireen Atabaki, M.D., Emergency Medicine (Joint membership with Center for Translational Science)
- Nancy Bauman, M.D., Otolaryngology
- Charles Berul, M.D., Cardiology
- Jaepyeong Cha, Ph.D.
- Kevin Cleary, Ph.D.
- Laurie Conklin, M.D., Gastroenterology (Joint membership with Center for Genetic Medicine Research)
- Adré du Plessis, M.D., Fetal and Transitional Medicine (Joint membership with Center for Neuroscience Research)
- Julia Finkel, M.D., Anesthesiology and Pain Medicine
- Michael Hsieh, M.D., Ph.D., Urology
- Timothy Kane, M.D., Minimally Invasive Surgery
- Joshua Kanter, M.D., Interventional Cardiology
- Aerang Kim, M.D., Ph.D., Oncology
- Anita Krishnan, M.D., Cardiology
- Marius Linguraru, Ph.D.
- Evan Nadler, M.D., Bariatric and General Surgery (Joint membership with Center for Genetic Medicine Research)
- Matthew Oetgen, M.D., Orthopaedic Surgery
- Albert Oh, M.D., Plastic and Reconstructive Surgery
- Laura Olivieri, M.D., Cardiology
- Hans Pohl, M.D., Urology (Joint membership with Center for Genetic Medicine Research)
- Nikki Posnack, Ph.D.
- Diego Preciado, M.D., Ph.D., Otolaryngology (Joint membership with Center for Genetic Medicine Research)
- Brian Reilly, M.D., Otolaryngology
- Anthony Sandler, M.D., General Surgery
- Karun Sharma, M.D., Ph.D., Radiology
- Raj Shekhar, Ph.D.
- Pavel Yarmolenko, Ph.D.
Individual Research Project Summaries
Body-Mounted MRI-Compatible Robot for Percutaneous Needle Procedures
- Kevin Cleary, Ph.D.
- Karun Sharma, M.D., Ph.D.
- Reza Monfaredi, Ph.D.
Minimally invasive procedures such as biopsy, drainage, or ablation (removal of tissue) are typically done under x-ray imaging to enable visualization of the site. Moving those procedures to the MRI environment could eliminate the radiation dose that occurs with x-ray imaging. The program is developing a body-mounted needle-positioning robot that is MR compatible. The first clinical application has focused on shoulder arthrography (visualization of the shoulder joint). This application was recently funded by a four-year NIH grant. The team developed a four-degree-of-freedom, patient-mounted robot to enable procedures in the MRI environment. Preliminary results in the MR environment show the distortion profile introduced by the robot is minimal. The goal of the next year is to complete phantom studies and move to clinical trial.
Robotically Assisted Rehabilitation for Children with Cerebral Palsy
- Sally Evans, M.D.
- Kevin Cleary, Ph.D.
- Catherine Coley, PT
- Reza Monfaredi, Ph.D.
- Hadi Fooladi
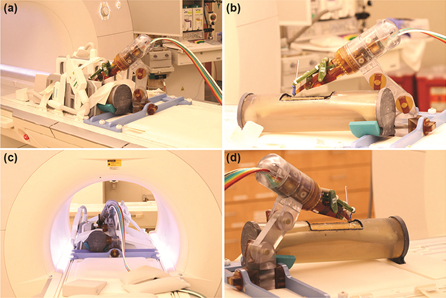
a) Robot mounted on table with long bone phantom in middle and imaging coils on both sides. (b) Coils removed to show phantom, robot, and long thin fiducial in needle guide (cutout in phantom. (c) Robot in scanner isocenter. Robot can be actuated to align needle guide while at isocenter. (d) View from right side illustrating robot mount and simulated positioning between legs.
More than half of the children with cerebral palsy have a gait disorder. The team has developed a new robotic motion platform with three degrees of freedom to exercise the ankle joint. The motion platform is connected to an airplane video game for which the patient’s foot controls the plane and points are awarded if the plane is flown successfully through moving hoops.
Non-invasive, Intraoperative Visualization of Critical Anatomic Structures Using Multimodal Optical Imaging Techniques
- Richard Jaepyeong Cha, Ph.D.
- Aline Broch, M.D.
- Scott Mudge
- Ki Hoon Kim, M.D., Ph.D.
- Jung-Man Namgoong, M.D.
- Peter Kim, M.D., Ph.D.
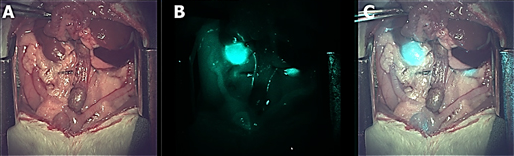
Novel fluorescent dyes for organ-specific visualization of biliary and urinary systems
Accurate, real-time identification and display of critical anatomic structures, such as the nerve and vasculature structures, are critical for reducing complications and improving surgical outcomes. Human vision is frequently limited in clearly distinguishing and contrasting these structures. The team introduced a novel imaging system, which enables noninvasive visualization of critical anatomic structures during surgical dissection. Peripheral nerves are visualized by a snapshot polarimetry that calculates the anisotropic optical properties. Vascular structures, both venous and arterial, are identified and monitored in real-time using a near-infrared laser-speckle-contrast imaging. The team has evaluated the system by performing in vivo animal studies with qualitative comparison by contrast-agent-aided fluorescence imaging.
Smart Tissue Automation Robot
- Axel Krieger, Ph.D.
- Peter Kim, M.D., Ph.D.
- Azad Shademan, Ph.D.
- Justin Opfermann
- Ryan Decker
- Simon Leonard, Ph.D.
- Hanh Le, Ph.D. Candidate (JHU)
- Jin Kang, Ph.D. (JHU)
The Smart Tissue Automation Robot (STAR) will help create smart surgical tools that are programmed with the best practices and techniques of experienced surgeons to consistently deliver optimal efficiency, effectiveness and safety. Anastomosis, the surgical connection made between adjacent blood vessels, parts of the intestine, urologic system or other channels of the body, is a critical task performed millions of times each year. Up to 30 percent of GI anastomoses, however, are complicated by leakage, strictures, and stenosis, in part attributable to technical and technologic issues of surgical tools. The team introduced three novel innovative technologies in STAR: 1) an end effector (a device at the end of a robotic arm, designed to interact with the environment) that incorporates and simplifies current surgical technique; 2) a new visual modality that allows tracking of mobile deformable soft tissue targets; and 3) collaborative decision support for surgical tasks between the surgeon and smart tools based on real-time target information. This paradigm of an “intelligent tool” exemplifies the next generation of surgical tools that will enhance the function and outcome of surgical tasks such as anastomosis.
Noninvasive Growth Plate Ablation
- Matthew Oetgen, M.D.
- Harry Kim, M.D.
- Pavel S. Yarmolenko, Ph.D.
- Haydar Celik, Ph.D.
- Olumide Aruwajoye, Ph.D.
- AeRang Kim, M.D., Ph.D.
- Karun Sharma, M.D., Ph.D.
- Kavita Prakash, BS
- Avinash Eranki, MS
- Robert Staruch, Ph.D.
- Rajiv Chopra, Ph.D.
- Peter Kim, M.D., Ph.D.
Pediatric leg length discrepancy (LLD) affects up to 40 percent of children, with up to 8percent having serious health effects. Unaddressed, the condition can cause a limp, lower back pain, scoliosis, poor posture, osteoarthritis of the hip and spine and other conditions. Treatment of LLD in children involves properly timed surgical destruction of the physis of the longer limb (epiphysiodesis) with different techniques in order to halt bone growth. These are invasive techniques that require a lengthy recovery, protected weight bearing and use of ionizing radiation for intraoperative guidance. Thus, a clear need exists for a non-invasive treatment method for LLD that minimizes both surgical trauma and exposure to ionizing radiation. The purpose of this study is to evaluate the utility of magnetic resonance imaging-guided high-intensity focused ultrasound (MR-HIFU) as a noninvasive treatment of LLD. The team’s initial animal study suggests that MR-HIFU can non-invasively ablate growth plate cartilage and has the potential to decrease complications and morbidities associated with traditional surgical methods. Further studies are warranted to develop a specific HIFU device for lower extremity use which is optimized, safe and effective for growth plate ablation.
MR-HIFU in the Treatment of Pediatric Tumors
- AeRang Kim, M.D., Ph.D.
- Karun Sharma, M.D., Ph.D.
- Pavel S. Yarmolenko, Ph.D.
- Matthew Oetgen, M.D.
- Haydar Celik, Ph.D.
- Avinash Eranki
- Ari Partanen, Ph.D.
- Peter Kim, M.D., Ph.D.
Despite intensification of therapy, survival has not significantly improved for metastatic and recurrent pediatric cancers over the past three decades. Recent advances in MR-HIFU have the potential to change cancer treatment paradigms by overcoming the primary limits of current therapies that are beset by side effects of aggressive treatment. The spatial accuracy and precision based on real-time imaging and temperature monitoring, lack of ionizing radiation, ability to release toxic chemotherapy only at the location of the tumor, and the noninvasive nature of MR-HIFU make it an extremely attractive modality to incorporate into existing treatment regimens for both first-time and recurrent solid tumors. The team’s ongoing Phase I clinical trial is the first to evaluate the safety and feasibility of MR-HIFU for pediatric malignant solid tumor ablation. The team is also investigating a combination of MR-HIFU with chemotherapeutic delivery via thermosensitive liposomes in a separate Phase I clinical trial. As a part of this trial, the group recently became the first to use this drug-device combination to treat a pediatric patient with a recurrent malignant solid tumor. In several pre-clinical studies, the team is investigating the possibility of improving the combined use of MR-HIFU with chemotherapy and its combination with immunotherapy to enhance antigen recognition in a metastatic murine solid tumor model.
Use of MR-Guided High Intensity Focused Ultrasound (MR-HIFU) for Non-Invasive Ablation of Osteoid Osteoma
- Karun V. Sharma, M.D., Ph.D.
- Pavel S. Yarmolenko, Ph.D.
- Haydar Celik, Ph.D.
- Avinash Eranki, MS
- Ari Partanen, Ph.D.
- Anilawan Smitthimedhin, M.D.
- Aerang Kim, M.D., Ph.D.
- Matthew Oetgen, M.D.
- Domiciano Santos, M.D.
- Janish Patel, M.D.
- Peter Kim, M.D., Ph.D.
Osteoid osteoma is a painful bone tumor occurring in children and young adults. Magnetic resonance imaging-guided high intensity focused ultrasound (MR-HIFU) allows non-invasive treatment without ionizing radiation exposure, in contrast to the current standard of care treatment with radiofrequency ablation (RFA). The group recently completed a prospective, nonrandomized multicenter clinical trial designed to evaluate the safety and tolerability of MR-HIFU ablative therapy in children with osteoid osteoma (benign bone tumor). This pilot study shows that MR-HIFU treatment of osteoid osteoma refractory to medical therapy is feasible and can be performed safely in pediatric patients. Clinical response is comparable with standard of care treatment but without any incisions or exposure to ionizing radiation.
Characterization of Histotripsy on a Clinical MR-Guided High Intensity Focused Ultrasound System
- Avinash Eranki, MS
- Navid Farr, Ph.D.
- Ari Partanen, Ph.D.
- Karun Sharma, M.D., Ph.D.
- Matthew Oetgen, M.D.
- Aerang Kim, M.D., Ph.D.
- Pavel S. Yarmolenko, Ph.D.
- Bradford Wood, M.D.
- Peter Kim, M.D., Ph.D.
While continuous-wave HIFU thermally ablates target tissue, the objective of this project was to characterize sonication parameter-dependent thermomechanical bioeffects of pulsed applications of HIFU. Effects of such sonications may include both thermal and mechanical tissue damage. The effect of choice of various pulsed sonication parameters on the type and extent of targeted tissue damage was not available in literature. Therefore, the team characterized lesion volume, temperature distribution, and area of lethal thermal dose for varying sonication parameters in tissue-mimicking phantoms and in ex vivo tissues. Thermomechanical HIFU bioeffects produced distinct types of fractionated tissue lesions: solid/thermal, paste-like and vacuolated. The results guide HIFU research on thermomechanical tissue bioeffects, inform future studies and advice sonication parameter selection for direct tumor ablation or immunomodulation using a clinical MR-HIFU system.
Cardiac Three-Dimensional Printing
- Laura Olivieri, M.D.
- Tom Loke, M.D.
- Justin Opfermann, MS
- Paige Mass
Accurate display of cardiac defects is critically important for clinical care, decision making, and surgical planning. The defect can be imaged using MRI, CT, or echocardiograph (echo) images. Despite the rich three-dimensional (3D) information provided by cardiac imaging, the display of this information is still largely constrained to viewing multiple contiguous two-dimensional (2D) slices of the 3D scan, which is suboptimal. The team is interested in demonstrating that surgical preparation before surgical correction for structural and congenital heart defects can be improved using 3D printed replicas of the patient’s heart anatomy. To date, more than 50 MR and 3D echo data sets have been obtained and successfully printed. The team is currently evaluating the effect of these models on clinical care and clinician education, both in formal didactics and in just-in-time simulation to successfully anticipate and manage the postoperative course. The group is spearheading a multicenter clinical study to determine the effect of printed models on surgical parameters (such as blood loss and bypass time) and outcomes.
New Technology for Determining Placental Function and Health
- Peter Kim, M.D., Ph.D.
- John Fisher, Ph.D.
- Che-Ying (Vincent) Kuo
- Navein Arumugasaamy
- Melissa Fries, M.D.
- Avinash Eranki
Preeclampsia (PE) is a leading cause of significant maternal and perinatal morbidity and mortality, affecting up to 8 percent of all pregnancies and contributing to greater than 60,000 maternal deaths worldwide each year. PE significantly affects fetal development. The only effective treatment is premature delivery of the fetus and placenta, resulting in significant fetal morbidity. The exact molecular and cellular mechanisms of how the trophoblast, the precursor of the placenta, invades and remodels the spiral arteriole (which provides its blood supply) are not known, and there is a paucity of relevant and suitable experimental models to study the mechanisms in human pregnancy. The team recently demonstrated for the first time that the unique geometric pattern of spiral arterioles can be bioprinted in a cell-laden placenta model, coated with endothelial cells, and perfused using the team’s 3D printed, perfusion-based bioreactor system. Using this bioprinted placenta model (BPM), the chemotactic invasion of trophoblasts can be engineered and quantified. This innovative approach offers a unique and effective way to study the normal human biology and abnormal pathology of the placenta.
Noninvasive Kidney Quantification for Hydronephrosis: Computer-Aided Diagnosis Tool (KidCAD)
- Marius George Linguraru, DPhil
- Pooneh Roshani Tabrizi, Ph.D.
- Antonio R. Porras, Ph.D.
- Hans Pohl, M.D.
- Dorothy Bulas, M.D.
When hydronephrosis (swelling of the kidneys) is found with ultrasound in children, the patient is often required to undertake an invasive and ionizing exam to determine the severity of hydronephrosis. The KidCAD project works to characterize hydronephrosis more precisely, noninvasively, and without radiation. The team developed new ultrasound-based quantitative imaging biomarkers of pediatric hydronephrosis and used machine learning to limit the need for ionizing exams in 62 to 85 percent of young patients. The need for surgical intervention can be predicted early to 93 percent accuracy using intelligent computational techniques. Support for the project is provided by Philips Healthcare.
Quantitative Volumetric Analysis of Optic Pathway Gliomas
- Marius George Linguraru, DPhil
- Awais Mansoor, Ph.D.
- Robert Avery, DO
- Roger Packer, M.D.
Nearly 20 percent of children with neurofibromatosis type 1 (NF-1) will develop an optic pathway glioma (OPG). About half of these children will experience vision loss. The team is developing and validating automated quantitative MRI analysis of the optic pathway in these children, demonstrating for the first time that the volume of NF-1- OPG strongly correlates with axonal degeneration in the retina and vision loss. Measuring the tumors in a precise, systematic manner, along with knowing how they grow, is the first step in recognizing which children are at highest risk for vision loss and to potentially identifying early intervention opportunities. The project is supported by the Gilbert Family Neurofibromatosis Institute.
Proteomic Networks of Inflammatory MUC5B Induction in Otitis Media (OM)
- Diego Preciado, M.D., Ph.D.
- Stephanie Val, Ph.D.
- Anna Kreuger
The team used a middle-ear cell culture system to understand the molecular pathobiology of OM progression, as well as to further characterize the proteomics of chronic otitis media. The team completed a proteomic profiling time series in vitro approach, identifying key pathways on the effects of nontypeable Haemophilus influenzae (HNTHi) and other pathogens on OM progression. The team discovered that mucoid middle-ear effusions from patients contain a preponderance of MUC5B expression.
The Role of Tympanostomy Tubes in Recurrent Otitis Media (rOM)
- Diego Preciado, M.D., Ph.D.
- Radhika Joshi
The efficacy of tubes for preventing rOM, assumedly by maintaining middle-ear ventilation, remains unclear. Limited evidence suggests short-term benefits similar in magnitude to those of antimicrobial prophylaxis. An advantage of tubes is that acute OM in children with tubes can be treated with topical rather than systemic antibiotics, potentially minimizing adverse effects and contributions to bacterial resistance. Benefits of tubes, however, must be balanced against risks of anesthesia and of the post-surgical development of discharge, blockage of the tube, or premature extrusion or displacement of the tube into the middle-ear cavity. Thus, a critical need exists to establish tympanostomy tubes’ risk/benefit ratio. The research project aims to determine the efficacy of tympanostomy tubes in children ages 6 to 35 months, the group in whom rAOM is most troublesome. To date, the team has recruited 100 patients into a randomization study to examine the risk/ benefit ratio of middle-ear tubes.
A Preclinical Trial Assessing the Efficacy and Safety of a Novel Bioresorbable Tracheal Stent
- Diego Preciado, M.D., Ph.D.
- Holly Rataiczak, M.D.
The management of laryngotracheal stenosis in children often poses a challenging problem to treating clinicians. Although success rates in achieving decannulation or avoiding tracheotomy of approximately 90 percent can be expected with open airway reconstructive procedures for the majority of cases and techniques, those success rates are lower in patients with severe or long-segment stenosis. The use of stents may play an important role in the surgical management of those patients. Currently, in cases of surgical failure, children frequently are condemned to live with tracheotomy tubes indefinitely. This is especially true with surgical failure of tracheal stenosis repair because revision surgery of the thoracic trachea is exceedingly challenging and risky. In those cases, usage of an indwelling, balloon-deployable stent could offer the benefit of providing an adequate airway while avoiding tracheotomy. The pediatric use of indwelling tracheal stents has also been proposed for cases of severe congenital or acquired tracheobronchomalacia. Unfortunately, because of a high rate of long-term complications and with difficulties associated with stent removal, the role of indwelling tracheal stents as a sole or adjuvant treatment of benign stenotic lesions is limited and has not been widely adopted. Over the past year, the group has been working in close collaboration with a medical device bioengineering company, Medical MurrayTM, toward the development of a polyglactin- based, bioresorbable, balloon-deployable, pediatric tracheal stent. A prototype stent and deployment system has been manufactured and has been tested in rabbits for longitudinal performance over six months. The team hypothesizes that the application of this novel indwelling bioresorbable tracheal stent will prove to be safe and effective for the treatment of tracheal narrowing.
Medical Device Biocompatibility
- Nikki Posnack, Ph.D.
- Luther Swift, Ph.D.
- Rafael Jaimes, Ph.D.
- Manelle Ramadan
- Bryan Siegel, M.D.
- Naomi Luban, M.D.
- An Massaro, M.D.
- Richard Jonas, M.D.
- Charles Berul, M.D.
- Billie Short, M.D.
- Khodayar Rais-Bahrami, M.D.
Medical Device Biocompatibility investigates the influence of biomaterial contaminants on cardiovascular and autonomic function, with an emphasis on plastic chemicals that can leach from medical devices, tubing, and blood storage containers. It is particularly relevant to neonates and young infants undergoing blood transfusions or complex reconstructive heart surgery. Any toxic effects of chemicals leaching from the various components of the cardiopulmonary bypass circuit or ECMO circuit are likely to have a magnified effect on the vulnerable physiology of the immature neonate or young infant. The team aims to identify safer biomaterials, chemicals, and surface coatings for transfusion devices, circuitry, and blood banking. Results of these studies can provide the foundation for objective decision making by scientific, medical and regulatory communities regarding the use of chemical additives in medical device manufacturing.
Pediatric Cardiac Research Models
- Nikki Posnack, Ph.D.
- Luther Swift, Ph.D.
- Rafael Jaimes, Ph.D.
- Manelle Ramadan
- Nobuyuki Ishibashi, M.D.
- Richard Jonas, M.D.
- Charles Berul, M.D.
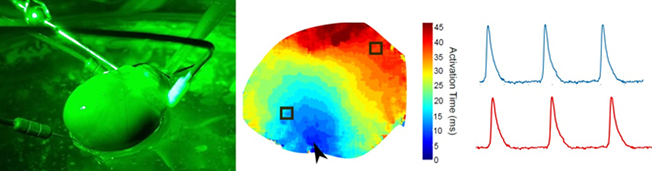
(A) Isolated, Langendorff-perfused whole rat heart. (B) Electrical conduction across the epicardial surface. (C) Optically mapped action potentials.
Pediatric cardiac research can be slowed by a shortage of appropriate human cardiac models. Human cardiomyocytes have a defined lifespan and do not readily replicate in culture. Moreover, immortalized human cardiomyocyte cell lines are non-contractile and lack both myofibril organization and a physiologically relevant action potential. Consequently, cardiovascular researchers often rely on rodent models—despite known species differences in myocardial structure and phenotype. To circumvent those hurdles, this laboratory uses human embryonic stem cell (hESC), human induced pluripotent stem cell (hiPSC), and piglet whole heart models. These models exhibit a phenotype that closely resembles fetal and neonatal human cardiac physiology and structure. The team employs these human models to predict pharmacological, toxicological and biocompatibility risk outcomes.
Pharmacological Agents and Cardioprotection
- Nikki Posnack, Ph.D.
- Rafael Jaimes, Ph.D.
- Luther Swift, Ph.D.
- Jeffrey Moak, M.D.
- Nobuyuki Ishibashi, M.D.
- Kamil Sarkislali, M.D.
Postoperative arrhythmias are a common occurrence in patients undergoing reconstructive heart surgery. Clinical studies have shown that magnesium (Mg2+) administration may decrease the incidence of post-operative arrhythmias in pediatric patients undergoing heart surgery. The team is investigating the cardioprotective effects of Mg2+ administration on excitation-contraction coupling, cardiac metabolism, and recovery from ischemia-reperfusion injury. Experimental procedures involve phenotypic measurements of whole heart tissue using a high-speed optical mapping system. Results of those studies can provide clinical guidance to optimize the administration of Mg2+ as a prophylactic intervention.
Vaccine Therapy for Cancer
- Anthony Sandler, M.D.
- Xiaofang Wu, Ph.D.
- Priya Srinivasan, Ph.D.
- Mousumi Basu
The team explored the use of attenuated live tumor cells as a method for optimal tumor antigen presentation and determined the effectiveness of combining antigen presentation with an immune activating agent (checkpoint blockade). The inhibitor of differentiation protein 2 (Id2) is found to be a key molecule modulating phenotypic transition in neuroblastoma. Knocking down Id2 confers immunogenicity to neuroblastoma tumors in immune competent hosts. Programmed cell death ligand-1 (PD- L1) expressed on tumors interferes with tumor-infiltrating lymphocytes through its interaction with programmed cell death-1 (PD1) present on the surface of immune cells. The team showed that immunogenic mouse neuroblastoma acquires adaptive immune resistance by up-regulating PD-L1, whereas PD-L1 is of lesser consequence in non- immunogenic tumors. Combining PD-L1 checkpoint inhibition with whole tumor cell/anti-CTLA4 vaccination enhanced tumor cell killing, cured all mice with established tumors, and induced long-term immune memory. This demonstrated the critical role PD-L1 plays in neuroblastoma’s resistance to immunity and defines the non- redundant effect of combination checkpoint inhibition with vaccine therapy.
Blow Spin Polymer Surgical Sealants
- Anthony Sandler, M.D.
- Peter Kofinas, Ph.D.
- John L. Daristotle Ph.D.
- Priya Srinivasan, Ph.D.
- Lung Wai Lau M.D.
Tissue reconstruction and closure of incisions and wounds is pertinent to almost all surgical interventions and traumatic injuries. This project is developing a medical sealant that reduces cost, inflammatory response and disease transmission risk, while improving procedural outcome. Current clinical options suffer from a combination of high cost, poor material properties, and biocompatibility issues. With the solution blow spinning technique, polymer fibers can be deposited directly onto any surface. This has the potential to become a powerful tool in surgery and in biomaterials fabrication. This proposal investigates the use of solution blow spun polymer blends of poly (ethylene glycol) (PEG) and poly (lactic-co-glycolic acid) (PLGA)) as a surgical sealant. This program utilizes the complementary expertise and facilities at the Sheikh Zayed Institute for Pediatric Surgical Innovation at Children’s National and the University of Maryland’s Functional Macromolecular Laboratory (UMD). Successful development of the direct deposition of polymer fiber constructs using solution blow spinning onto any surface could lead to clinically translatable approaches in a diverse variety of surgical applications and in biomaterials fabrication.
mGene: Early Mobile Detection of Genetic Syndromes
- Marius George Linguraru, DPhil
- Antonio R. Porras, Ph.D.
- Liyun Tu, Ph.D.
- Marshall Summar, M.D.
- Eric Vilain, MD Ph.D.
- Matthew Bramble, Ph.D.
One million children every year are born in the world with a chromosomal abnormality. Children with chromosomal abnormalities have a high incidence of intellectual disability, as well as serious medical complications (cardiac, pulmonary, motor) that require treatment and often surgery. Because of these related complications, it is critical to detect genetic syndromes early. The team developed mGene, a software technology that can assess neonates and infants without the need for blood tests or specialized clinics. This noninvasive test uses automated facial recognition as a screening tool and can make the detection of genetic syndromes as easy as a snapshot. Multi-institutional validation is ongoing in Washington, D.C., and through collaborations with the NIH National Human Genome Research Institute and health and research authorities of the United Arab Emirates and the Democratic Republic of the Congo.
Image-Guided Planning System for Cranial Correction in Children with Craniosynostosis
- Marius George Linguraru, DPhil
- Antonio R. Porras, Ph.D.
- Liyun Tu, Ph.D.
- Gary Rogers, M.D., MBA
- Robert Keating, M.D.
- Albert Oh, M.D.
Craniosynostosis is the premature fusion of cranial sutures (fibrous joints) and occurs in approximately one in 2,000 births. It results in a cranial malformation that can lead to elevated intra-cranial pressure, brain growth impairment, and intellectual disability. The most common treatment option for craniosynostosis is surgery; however, the surgical treatment planning of craniosynostosis is currently qualitative and irreproducible. The team is developing and evaluating intelligent cranial surgical planning (iCSPlan) software technology that enables optimal and personalized cranial remodeling in children with craniosynostosis. iCSPlan allowed the team to define the first quantitative and repeatable clinical criterion to diagnose metopic craniosynostosis. The team is developing automated surgical planning for optimal osteotomy and bone placement during surgery and objective evaluation of surgical outcomes. The project is supported by an NIH Phase II Small Business Technology Transfer (STTR) grant, in collaboration with Kitware, Inc.
Augmented Reality (AR) Visualization for Laparoscopic Surgery
- Raj Shekhar, Ph.D.
- Timothy Kane, M.D.
- Karun Sharma, M.D., Ph.D.
- Xinyang Liu, Ph.D.
- Lung Lau, M.D.
- William Plishker, Ph.D.
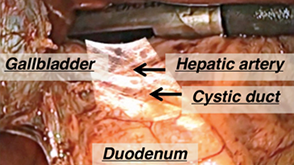
Real-time ultrasound data overlaid on live laparoscopic video during a laparoscopic cholecystectomy case (gall bladder removal).
Laparoscopic surgery is an attractive alternative to conventional open surgery and is known to improve outcomes, cause less scarring, and lead to significantly faster patient recovery. Standard laparoscopy cannot visualize anatomy beyond what is present in front of the laparoscope. Conventional laparoscopic ultrasound imaging provides information on subsurface anatomy, but can only be integrated with the laparoscopic images in the surgeon’s mind. Additionally, focus is distracted from the laparoscopy screen to look at ultrasound images on a separate screen. The team developed an augmented reality (AR) system that fuses real-time ultrasound images with live laparoscopic video, allowing explicit visualization of subsurface structures (blood vessels, bile ducts, tumors, etc.) in the surgical field of view. Potential applications of this system include laparoscopic partial hepatectomy (liver resection) and laparoscopic radiofrequency ablation of liver cancer. A first generation prototype has been tested on 13 human cases so far at Children’s National. A second generation prototype is being tested on animals (swine) and will be evaluated at Children’s National and another adult surgical center (University of Pittsburgh Medical Center).
StethAid: Automated Point-of-Care Identification of Innocent Still’s Murmur in Children
- Raj Shekhar, Ph.D.
- Robin Doroshow, M.D.
- Titus John, MS
- Ravi Ambati, MS
An estimated half a million children in the United States are referred to pediatric cardiologists by their pediatricians each year for the evaluation of a heart murmur. In approximately 80 percent of these children, the murmur turns out to be Still’s murmur, an innocent (benign) heart murmur of childhood. These unnecessary referrals and associated tests cost the health care system over half a billion annually and are a source of avoidable anxiety among children and their parents. The team’s solution, StethAid, is a mobile-device-based digital stethoscope accompanied with a highly accurate machine learning algorithm to differentiate Still’s murmur from all other murmurs including pathological murmurs. The focus of the team’s ongoing research and development is to create a validated mobile technology for identifying Still’s murmur in the primary office setting. As a decision support system, StethAid could empower pediatricians to identify Still’s murmur accurately and thus reduce the large number of unnecessary pediatric cardiologist referrals. This should save the health care system hundreds of millions of dollars annually, allow pediatric cardiologists to focus on patients with serious conditions, and protect healthy children and their families from the unnecessary anxiety, inconvenience, and expense of seeing a pediatric cardiologist.
Minimally Invasive Pacemaker/Defibrillator
- Charles Berul, M.D.
- Justin Opfermann
- Bradley Clark, M.D.
- Rohan Kumthekar
- Paige Mass
In pediatric and adult patients with complex congenital heart disease, standard transvenous pacemaker and defibrillator placement is not a viable option. The only currently available alternative is open-chest placement of pacing leads directly on the heart, a significantly invasive procedure. The team is presently developing minimally invasive percutaneous lead delivery tools and techniques for implanting pacemaker and defibrillator leads via a pericardiocentesis needle to access the heart, specifically designed for pediatric and congenital heart applications. To demonstrate safety and feasibility of the percutaneous technique, the team’s most recent device, PeriPath, is undergoing preclinical testing using an infant piglet model.
Optical Imaging and Characterization of the Human Middle Ear with Otitis Media Using an Optical Coherence Tomography (OCT)-Based Otoscope
- Diego Preciado, M.D., Ph.D.
- Nancy Bauman, M.D.
- Radhika Joshi
This is an observational imaging study, funded by National Institute on Deafness and Other Communication Disorders (NIDCD) through an SBIR mechanism in collaboration with Photonicare, Inc., whereby pediatric subjects are being recruited when visiting their pediatrician or otolaryngologist at Children’s National. Non-invasive, label-free imaging was performed with a commercial prototype of a portable clinical optical coherence tomography (OCT) system with a handheld probe. Bilateral high-resolution (5-15 micron), cross-sectional imaging of tympanic membrane (TM) and middle-ear contents and corresponding clinical diagnoses and histories were collected. Blinded analysis of correlated OCT and video otoscope images was conducted for the presence of middle-ear content, specifically effusion. Preliminary results suggest the cross-sectional imaging capabilities of OCT may improve physician assessment and diagnosis of middle-ear infection.
Pupil-Algometer
- Julia Finkel, M.D.
- Kevin Jackson
- Christina Shincovich
- Luka Vujaskovic
The pupil-algometer is a device and method designed to measure pain intensity and type and guide analgesic drug delivery in verbal and nonverbal patients. The device integrates a smartphone-enabled infrared camera, for IOS and Android, to image the eye (pupillometer), along with a neuro-specific neurostimulator that allows for the determination of pain type and sensitivity. The smartphone device measures pupillary light reflex (PLR) and pupillary reflex dilation (PRD), incorporating proprietary clinical algorithms with point-and-shoot pupillary imaging. The software designed will be a HIPAA-compliant, cloud-based, patient engagement platform. The device and methods being developed will assist in diagnosis and selection of best pain treatment options.
Selected Publications from 17/18
- Clark BC, Opfermann JD, Davis TD, Krieger A, Berul CI., 2017, ìSingle-incision percutaneous pericardial ICD lead placement in a piglet model,ì J. Cardiovasc Electrophysiol, Jun 1. doi:10.1111/jce.13263. [Epub ahead of print]
- Decker, RS, Shademan A, Opfermann JD, Leonard S, Kim PCW and Krieger, A, "Biocompatible Near-Infrared Three-Dimensional Tracking System," in IEEE Transactions on Biomedical Engineering, vol. 64, no. 3, pp. 549-556, March 2017. doi: 10.1109/TBME.2017.2656803.
- PD-L1 checkpoint inhibition and anti-CTLA-4 whole tumor cell vaccination counter adaptive immune resistance: A mouse neuroblastoma model that mimics human disease. Srinivasan P, Wu X, Basu M, Rossi C, Sandler AD. PLoS Med. 2018 Jan 29;15(1):e1002497. doi: 10.1371/journal.pmed.1002497. eCollection 2018 Jan.
- MYCN Amplification Is Associated with Repressed Cellular Immunity in Neuroblastoma: An In Silico Immunological Analysis of TARGET Database. Zhang P, Wu X, Basu M, Dong C, Zheng P, Liu Y, Sandler AD. Front Immunol. 2017 Nov 3;8:1473. doi: 10.3389/fimmu.2017.01473. eCollection 2017.
- Reza Monfaredi, Hadi Fooladi, Pooneh Roshani, Staci Kovelman, Tyler Salvador, Catherine Coley, Sara Alyamani, Paola Pergami, Kevin Cleary, Sally Evans, "PedBot: robotically assisted ankle robot and video game for children with neuromuscular disorders", Proc. SPIE 10576, Medical Imaging 2018: Image-Guided Procedures, Robotic Interventions, and Modeling, 105761R (13 March 2018); doi: 10.1117/12.2295031; https://doi.org/10.1117/12.2295031
- Robotically Assisted Long Bone Biopsy Under MRI Imaging: Workflow and Preclinical Study. Kevin Cleary, PhD, Sunghwan Lim, MS, Changhan Jun, MS, Reza Monfaredi, PhD, Karun Sharma, MD/PhD, Stanley Thomas Fricke, NuclEng, PhD, Luis Vargas, RT (MR), Doru Petrisor, PhD, Dan Stoianovici, PhD
- Blum, E.S. Porras, A.R. Biggs, E. Tabrizi, P.R. Sussman, R.D. Sprague, B.M. Shalaby-Rana, E. Majd, M. Pohl, H.G. Linguraru, M.G.: Early detection of ureteropelvic junction obstruction using signal analysis and machine learning: A dynamic Solution to a dynamic Problem. Journal of Urology (2017) doi:10.1016/j.juro.2017.09.147
- Kruszka, P. Addissie, Y. McGinn, D. Porras, A.R. Biggs, E. Share, M. Crowley, T. Chung, B.H.Y. Mok, G.T.K. Muthukumarasamy, P. Thong, M.K. Sirisena, N. Dissanayake, V.H.W. Paththinige, C. Prabodha, L.B. Mishra, R. Shotelersuk, V. Ekure, E. Sokunbi, O. Kalu, N. Ferreira, C. Duncan, J.M. Patil, S. Jones, K. Kaplan, J. Abdul-Rahman, O. Gil-da-Silva-Lopes, V.L. Moresco, A. Obregon, M.G. Richieri-Costa, A, Adeyemo, A. Summar, M. Zackai, E. McDonald-McGinn, D. Linguraru, M.G. Muenke, M. : 22q11.2 Deletion Syndrome in Diverse Populations. American Journal of Medical Genetics Part A, Vol. 173(4) (2017) 879-888
- Kang S, Doroshow R, McConnaughey J, Shekhar R. Automated Identification of Innocent Still’s Murmur in Children. IEEE Transactions on Biomedical Engineering, 64(6):1326-1334, 2017.
- Liu X, Rice CE, Shekhar R. Fast calibration of electromagnetically tracked oblique-viewing rigid endoscopes. International Journal of Computer Assisted Radiology and Surgery, 12(10):1685-1695, 2017.
- Sharma KV, Yarmolenko PS, Eranki A, Partanen A, Celik H, Kim A, Oetgen M, Kim PC. Magnetic resonance imagingñguided high-intensity focused ultrasound applications in pediatrics: early experience at children’s national medical center. Topics in Magnetic Resonance Imaging. 2018 Feb 1;27(1):45-51.
- Eranki A, Farr N, Partanen A, Sharma KV, Chen H, Rossi CT, Kothapalli SV, Oetgen M, Kim A, Negussie AH, Woods D, Wood BJ, Kim PCW, Yarmolenko PS. Boiling histotripsy lesion characterization on a clinical magnetic resonance imaging-guided high intensity focused ultrasound system. PloS one. 2017 Mar 16;12(3):e0173867.